Advertisement|Remove ads.
Why Is Scienture Stock Rising Today?
- Scienture has commenced selling Arbli, the first FDA-approved ready-to-use liquid losartan formulation.
- The U.S. losartan market is worth about $256 million annually, offering strong growth potential for Arbli.
- Scienture secured key GPO agreements to expand Arbli’s availability to over 2,500 healthcare facilities nationwide.
Scienture Holdings Inc. (SCNX) on Thursday announced the launch of commercial sales for its new product, Arbli (losartan potassium) Oral Suspension, 10 mg/mL.
Arbli is the first FDA-approved, ready-to-use oral suspension of losartan potassium, offering patients who require alternatives to pills a safe and consistent treatment option.
With the U.S. losartan market generating around $256 million annually and more than 71 million prescriptions written each year, according to IQVIA data, Scienture sees a strong potential for this new product.
How Did Stocktwits Users React?
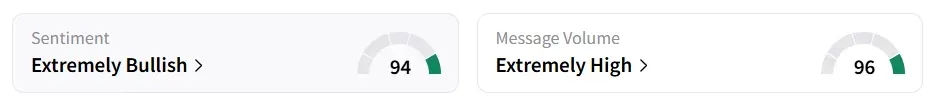
Shares of Scienture Holdings surged by more than 122% by Thursday noon. On Stocktwits, retail sentiment around the stock remained in ‘extremely bullish’ territory amid ‘extremely high’ message volume levels.
A bullish Stocktwits user said the company’s valuation might reach billions if the product is expanded globally.
Arbli’s Importance
Arbli is a unique liquid form of losartan, a common medication for high blood pressure. It doesn’t need compounding, offers a smaller dosing volume, and has a long shelf life at room temperature. Arbli is approved to treat hypertension in patients over six years old, to lower stroke risk in those with hypertension and heart enlargement, and manage diabetic kidney disease in certain type 2 diabetes patients.
To support Arbli’s market entry, Scienture has secured Pharmacy Benefit Manager-led Group Purchasing Organization (PBM-Led GPO) agreements to broaden commercial coverage and formulary inclusion. Scienture has also finalized contracts with multiple commercial GPOs, allowing Arbli to reach over 2,500 healthcare institutions nationwide.
Scienture’s stock has lost over 79% in 2025 and over 82% in the last 12 months.
Also See: Datavault AI To Boost Growth With New Headquarters, Quantum Centers
For updates and corrections, email newsroom[at]stocktwits[dot]com.









/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_140648412_jpg_05552b4117.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/Anushka_Basu_make_me_smile_in_the_picture_b92832aa_af59_4141_aacc_4180d2241ba8_1_2_png_1086e0ed8c.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_912727778_jpg_3bd44c57ad.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_1235943608_jpg_91702ef0ab.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_167578473_jpg_29255f031f.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Michael_Burry_jpg_fa0de6ac92.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_winklevoss_1c7de389e0.webp)