Advertisement|Remove ads.
HIMS Stock Plunges 10% After Hours: FDA Chief Warns Of ‘Swift Action’ On Copycat Drugs After Novo Semaglutide Knockoff Launch

- The drop followed a same-day launch of a compounded semaglutide pill that drew FDA warnings and a legal pushback from Novo Nordisk.
- Novo said only its Wegovy capsule uses FDA-approved semaglutide with SNAC technology.
- Hims defended its approach, calling pharma criticism “predictable.”
Shares of Hims & Hers Health Inc. plunged 10% in extended trading on Thursday as the company's launch of a compounded Semaglutide pill drew pushback from the FDA and Novo Nordisk.
In the regular session, the stock fell nearly 4%, marking its fifth consecutive session of losses and its lowest levels in over 14 months.
FDA Warns Of ‘Swift Action’ Against Copycat Drugs
FDA Commissioner Marty Makary said on X that the "FDA will take swift action against companies mass-marketing illegal copycat drugs, claiming they are similar to FDA-approved products." He added that "The FDA cannot verify the quality, safety, or effectiveness of non-approved drugs."
Within hours of Hims’ announcement, Novo issued a statement, calling the move illegal mass compounding of an unapproved, inauthentic, and untested Semaglutide pill. The drugmaker said it would pursue legal and regulatory action to protect patients, its intellectual property, and the U.S. drug approval framework.
Novo noted that it is the only company producing an FDA-approved Wegovy capsule. That capsule contains Semaglutide formulated with SNAC technology that the company developed. Compounded Semaglutide, Novo said, has not been approved by the FDA and could contain impurities, unnecessary ingredients, or experimental doses. The company also cited new standards of care that discourage compounded GLP-1s due to safety and effectiveness concerns.
Hims Rolls Out Compounded Semaglutide Pill
Hims announced earlier Thursday that providers on its platform could begin prescribing compounded semaglutide pills as part of the telehealth startup's expanded weight-loss services. Marketed as a needle-free alternative for patients looking for more personalization around their weight-loss journey, the pill starts at $49 for the first month for qualified patients.
Hims said active pharmaceutical ingredients used in compounded treatments are sourced from FDA-registered facilities. It also acknowledged that compounded drugs are not approved or evaluated by the FDA for safety, effectiveness, or quality.
Hims Pushes Back On Criticism
Hims pushed back on X, saying, "Our track record speaks for itself and is why we’ve been able to help nearly 2.5M customers access care personalized to their needs. We’re focused on bringing more access, more positive outcomes, and more choice to customers everywhere."
Hims added that this was not the first time a large pharmaceutical company had claimed that its customer-first approach is dangerous or illegal. "This narrative is as predictable as it is outdated and false," the company said.
FDA Highlights Risks In Unapproved GLP-1 Drugs
In a separate release on Wednesday, the FDA said unapproved versions of GLP-1 drugs like Semaglutide and Tirzepatide skip FDA review of safety, effectiveness, and quality prior to marketing.
The FDA recommended that compounded GLP-1 products should only be used when necessary to meet the medical needs of a patient that cannot be met with an FDA-approved product. The agency also warned about issues regarding improper storage, dosing errors, and fraudulently sold products. As of July 31, 2025, the FDA says it has received 605 adverse event reports associated with compounded Semaglutide and 545 reports associated with compounded Tirzepatide. The agency notes that reports are likely underreported.
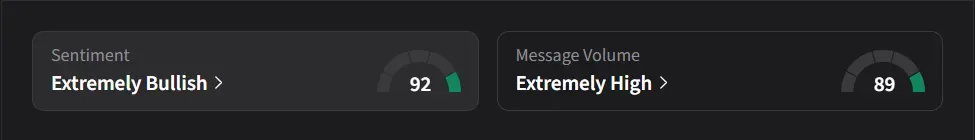
How Did Stocktwits Users React?
On Stocktwits, retail sentiment for Hims was ‘extremely bullish’ amid ‘extremely high’ message volume.
One user said, “This legal action will cost them a lot.”
Another user said the strategy is “going to look genius months from now,” pairing cancer detection with a cheaper Wegovy alternative and a Super Bowl ad calling out how healthcare has long favored the wealthy.
Hims’ stock has declined 44% over the past 12 months.
For updates and corrections, email newsroom[at]stocktwits[dot]com.











/filters:format(webp)https://news.stocktwits-cdn.com/large_stock_price_bitcoin_OG_jpg_d43a7edd5e.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/Prabhjote_DP_67623a9828.jpg)
/filters:format(webp)https://news.stocktwits-cdn.com/Getty_Images_2227994915_jpg_357c625963.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/IMG_8805_JPG_6768aaedc3.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_1470886126_jpg_f11dd80298.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/IMG_4530_jpeg_a09abb56e6.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2262161352_jpg_832967dbca.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/IMG_9209_1_d9c1acde92.jpeg)
/filters:format(webp)https://news.stocktwits-cdn.com/large_bitcoin_OG_oct23_jpg_588046d0a9.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_patrick_soon_shiong_jpg_5f4d6bc18d.webp)