Advertisement|Remove ads.
Rocket Pharma Stock Gets A Price Target Cut After Withdrawing FDA Application For Rare Bone Marrow Disorder Gene Therapy

Leerink lowered its price target on Rocket Pharmaceuticals (RCKT) to $7 from $9 on Friday, following the company's announcement that it has voluntarily withdrawn its application for the approval of Fanskya to the U.S. Food and Drug Administration for the treatment of Fanconi Anemia.
The analyst kept a ‘Market Perform’ rating on the shares, according to TheFly. The new price target, however, implies a potential upside of approximately 118% from the stock’s closing price on Thursday.
Rocket Pharma stated in a filing with the Securities and Exchange Commission that the decision to withdraw the application for the gene therapy follows its decision to focus its resources on programs with the clearest regulatory and commercial pathways. The decision is based on business and strategic considerations and does not reflect concerns regarding the safety or efficacy profile of Fanskya or RP-L102, the company said.
“Data generated to date continue to support that RP-L102 has been generally well tolerated, with no significant safety signals observed, and a risk-benefit profile that appears favorable,” it added.
The company ceased internal investment in Fanskya in July 2025 and withdrew its application with the European Medicines Agency (EMA) as part of a pipeline prioritization and corporate reorganization plan. The company had then warned that FDA approval of Fanskya is no longer expected in 2026.
The therapy was tested in the treatment of Fanconi Anemia, a rare, inherited disorder characterized by a failure of the bone marrow to produce enough blood cells, leading to symptoms like fatigue, bleeding, and infections, as well as physical abnormalities and a significantly increased risk of certain cancers.
The company said on Friday that it will consider external partnership opportunities to advance Fanskya in the future. “Withdrawal of the BLA preserves Rocket’s ability to re-engage with regulators at a later date should there be an appropriate strategic or partnership pathway to sustainably progress the program,” it said.
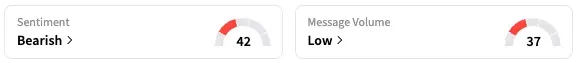
Shares of RCKT fell 2% at the time of writing. On Stocktwits, retail sentiment around RCKT remained within the ‘bearish’ territory while message volume remained at ‘low’ levels.
In August, Rocket Pharma said that the FDA had lifted the clinical hold on its mid-stage study of RP-A501 for the treatment of Danon disease. The hold, imposed roughly three months ago following the death of a patient in the trial, was lifted after Rocket satisfactorily addressed issues outlined in the clinical hold, the company said. The agency authorized the study to resume with an adjusted dose of the investigational gene therapy.
RCKT stock is down by 75% this year.
Read also: Alto Neuroscience Stock Soars After FDA Grants Fast Track Designation To Schizophrenia Drug
For updates and corrections, email newsroom[at]stocktwits[dot]com.












/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2229918735_jpg_e905cbd5e3.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/IMG_9209_1_d9c1acde92.jpeg)
/filters:format(webp)https://news.stocktwits-cdn.com/large_nio_stock_jpg_770e12377f.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/IMG_8805_JPG_6768aaedc3.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_rocket_companies_logo_resized_jpg_1cfb06fa99.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/IMG_4530_jpeg_a09abb56e6.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_corcept_therapeutics_jpg_0778e9d4e5.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_cryptocurrency_generic_jpg_4184e1dbd8.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/Prabhjote_DP_67623a9828.jpg)
/filters:format(webp)https://news.stocktwits-cdn.com/large_iova_stock_jpg_ac0924fcdd.webp)