Advertisement|Remove ads.
IBRX Stock Extends Rally On FDA Feedback For ANKTIVA Bladder Cancer Filing

- ANKTIVA is an Interleukin-15 (IL-15) receptor agonist that helps develop and activate key cells that work to destroy cancer cells.
- The company presented an overview of the clinical status of its papillary disease program, including more than five years of follow-up data.
- The data demonstrated strong bladder preservation outcomes.
ImmunityBio (IBRX) shares rose 6% in pre-market trading on Tuesday after the United States Food and Drug Administration (FDA) outlined a pathway for resubmitting its supplemental biologics license application (sBLA) for ANKTIVA in Bacillus Calmette Guérin (BCG)-unresponsive papillary bladder cancer, without requesting new trials.
The company said it recently met with the FDA for a Type B end-of-phase meeting to review data spanning over five years.
The FDA Meeting
During the meeting, ImmunityBio presented more than five years of follow-up data supporting the papillary disease indication. Long-term results, published in The Journal of Urology, showed bladder cancer–specific survival of around 96% at three years, with median survival not yet reached.
The data also demonstrated strong bladder preservation outcomes, with cystectomy avoidance rates of 92% at one year and 82% at three years, alongside a safety profile consistent with ANKTIVA’s currently approved use in carcinoma in situ (CIS).
Based on these discussions, the FDA recommended that ImmunityBio submit additional information for further evaluation to potentially support a resubmission of the sBLA initially filed in 2025. The Agency did not request any new clinical trials.
“We have completed the assembly and analysis of the requested additional information and will submit it within the next 30 days for the Agency’s review,” said Richard Adcock, President and CEO of ImmunityBio.
ANKTIVA is an Interleukin-15 (IL-15) receptor agonist. IL-15 is an immune system protein that helps develop and activate key cells that work to destroy cancer cells. It is already commercially available in the U.S. for NMIBC CIS, with or without papillary tumors, and has received approvals in the U.K. and Saudi Arabia, as well as conditional approval in the European Union.
How Did Stocktwits Users React?
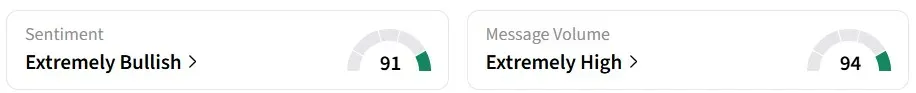
Retail sentiment on Stocktwits for IBRX remained in the ‘extremely bullish’ territory over the past 24 hours, amid ‘extremely high’ message volumes. IBRX was among the top trending tickers on the platform at the time of writing.
One user laid out the potential triggers for the stock.
Over the past year, the stock has gained 85,% and year-to-date, it has added more than 173%.
Read also: Why Did RAPT Stock Surge 63% In Pre-Market Today?
For updates and corrections, email newsroom[at]stocktwits[dot]com.











/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2033244575_jpg_3f112039eb.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/IMG_4530_jpeg_a09abb56e6.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2256076198_jpg_06e5c2fdb6.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/IMG_9209_1_d9c1acde92.jpeg)
/filters:format(webp)https://news.stocktwits-cdn.com/large_pharma_stock_jpg_490939e580.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/IMG_8805_JPG_6768aaedc3.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_stablecoin_rep_jpg_5ec196dfc2.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/Prabhjote_DP_67623a9828.jpg)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2228901342_jpg_7365e02c40.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2218742693_jpg_8d1b39840a.webp)