Advertisement|Remove ads.
Why Nano-Cap Adagio Medical Saw The Highest Weekly Spurt In Retail Following Among Healthcare Stocks

Shares of Adagio Medical Holdings, Inc. (ADGM) experienced a significant surge in retail investor interest last week as they gained over 122%, driven by regulatory approval for a medical device.
On Thursday, Adagio said its vCLAS Cryoablation System had been granted Breakthrough Device designation by the U.S. Food and Drug Administration (FDA) for the treatment of drug-refractory, recurrent, sustained monomorphic ventricular tachycardia (VT) in patients with structural heart disease.
CEO Todd Usen said the regulatory nod marked a significant milestone for Adagio as it validated its proprietary system as a "potentially unique solution for the large, underserved population of patients suffering from ventricular tachycardia."
"We are thrilled to be the only technology to be granted Breakthrough Device designation for endocardial treatment of both ischemic and nonischemic structural heart disease patients with sustained monomorphic VT," he added.
Adagio said the FDA's decision was partly based on clinical data from Adagio's European CRYOCURE-VT study.
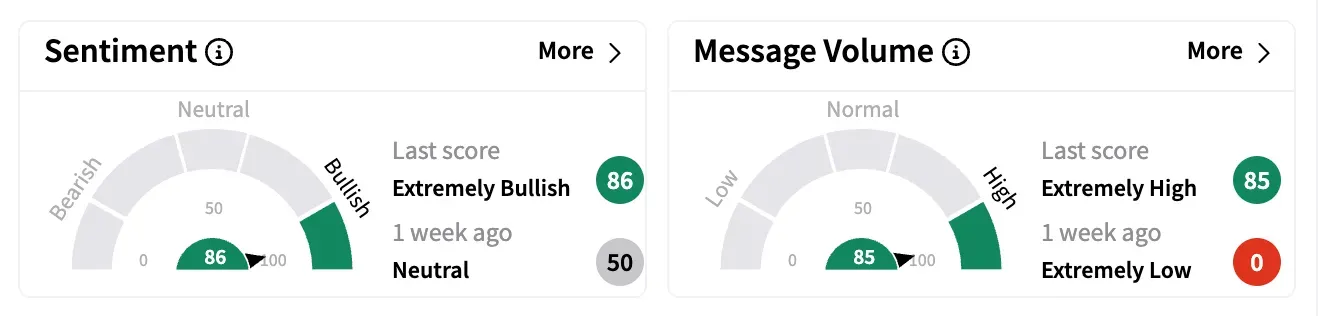
The news resulted in Adagio's retail following on Stocktwits jumping by over 64% last week, with message volume flipping to 'extremely high' levels and sentiment surging to 'extremely bullish.'
One user called the stock surge the "best setup for a major squeeze," although Koyfin data showed Adagio has a relatively low short interest of just 0.5%.
The free float, however, stands at just over 15 million.
Another believed Adagio would nearly triple on Monday.
Adagio's stock has gained more than 45% this year but is down over 84% in the past 12 months.
For updates and corrections, email newsroom[at]stocktwits[dot]com.












/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2263454468_jpg_23f4595a31.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/Anushka_Basu_make_me_smile_in_the_picture_b92832aa_af59_4141_aacc_4180d2241ba8_1_2_png_1086e0ed8c.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2264020227_jpg_4d7420bef3.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2259210190_jpg_d48bbe3269.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_1396534113_jpg_b0e09f299b.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Jane_Street_3ac3fb6443.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Truth_social_5bfbc7389b.webp)