Advertisement|Remove ads.
Axsome Stock Rockets To Record High After Settling With Teva Over Depression Drug, Retail Optimism Swells
Axsome Therapeutics (AXSM) shares surged over 15% to a record high Monday morning after the company reached a settlement with Teva Pharmaceuticals (TEVA), resolving all patent litigation related to its depression drug, Auvelity.
The stock is on track for its best session since November 28, 2022.
The litigation stemmed from Teva's Abbreviated New Drug Application (ANDA) to the FDA, seeking approval to market a generic version of Auvelity before Axsome's patents expired.
Under the settlement terms, Teva will receive a license to sell a generic version of Auvelity starting March 31, 2039, if pediatric exclusivity is granted.
If not, the license will begin six months earlier, on Sept. 30, 2038.
As part of the agreement, all ongoing litigation between Axsome and Teva in the U.S. District Court of New Jersey will be terminated.
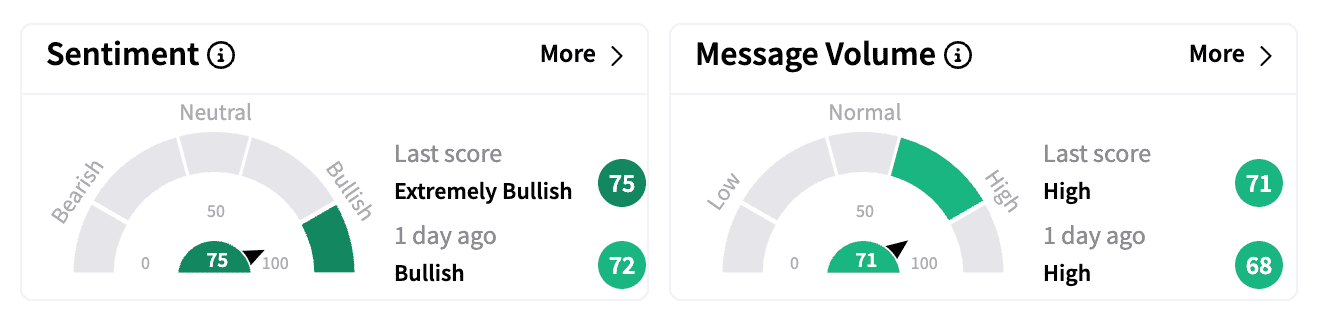
On Stocktwits, sentiment for Axsome's stock turned even more bullish as message volume spiked.
One user cheered the decision, saying: "Auvelity goes generic in the year 2039 (instead of 2034). That's BIG!"
Another said this was perhaps "the short squeeze we've been waiting months, if not years for."
According to Koyfin data, Axsome's stock has a short interest of 11.9%, which has reduced from 13.4% at the start of the year.
In December, shares dipped following mixed results in two Alzheimer's studies testing Auvelity for agitation related to the disease.
However, the company plans to file for FDA approval for an Alzheimer's agitation label in the second half of 2025, alongside a submission for AXS-12 in narcolepsy.
Axsome is also preparing for a potential launch of AXS-07 for acute migraine treatment, pending FDA approval.
The stock has gained nearly 25% over the past 12 months.
For updates and corrections, email newsroom[at]stocktwits[dot]com.












/filters:format(webp)https://news.stocktwits-cdn.com/large_william_li_nio_jpg_c26eaad557.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/IMG_8805_JPG_6768aaedc3.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_arthur_hayes_OG_jpg_734ff95af6.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/Prabhjote_DP_67623a9828.jpg)
/filters:format(webp)https://news.stocktwits-cdn.com/Getty_Images_1247400381_jpg_765e6ec016.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_strategy_logo_OG_jpg_fa4e1a7d04.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2206312585_jpg_1a7c050dff.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/IMG_9209_1_d9c1acde92.jpeg)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2227669377_jpg_9a115c3623.webp)