Advertisement|Remove ads.
Gilead Stock Gains After Twice-Yearly Shot Reduced HIV Infections By 96%: Retail Turns 'Extremely Bullish'

Gilead Sciences, Inc. (GILD), on Thursday, announced positive results from a clinical trial investigating the use of the company’s twice-yearly injectable Lenacapavir, sending its shares up by over 2%.
Gilead said that Lenacapavir reduced HIV infections by 96% and highlighted that 99.9% of the participants did not acquire HIV infection in the Lenacapavir group, with two incident cases among 2,180 participants.
The trial, PURPOSE 2, included cisgender men, transgender men, transgender women, and gender non-binary individuals in Argentina, Brazil, Mexico, Peru, South Africa, Thailand and the United States who have sex with partners assigned male at birth.
The PURPOSE trials are evaluating the safety and efficacy of Lenacapavir to reduce the chance of getting HIV. The program comprises five HIV prevention trials around the world.
Gilead said the data from the PURPOSE 1 and PURPOSE 2 trials will support upcoming regulatory filings so that twice-yearly Lenacapavir for PrEP, if approved, can be made available to multiple populations and communities around the world who are most in need of additional HIV prevention choices. PrEP is a medication used to prevent getting HIV.
The company is set to begin a series of global regulatory filings by the end of 2024 and said this could support the initial launch of the injectable in 2025.
Gilead also stated that it is executing an access strategy that prioritizes speed and enables the most efficient paths for the regulatory review and approval of Lenacapavir for PrEP in regions around the world. “This strategy will prioritize high-incidence, low-resource countries, which are primarily low- and lower-middle income countries,” it said.
The firm, however, clarified that the use of Lenacapavir for the prevention of HIV is investigational, has not been determined to be safe or efficacious, and is not approved anywhere globally. “There is currently no cure for HIV or AIDS,” it said.
Following the announcement, retail sentiment on Stocktwits climbed into the ‘extremely bullish’ territory (80/100) from ‘bullish’ a day ago, accompanied by ‘extremely high’ message volume.
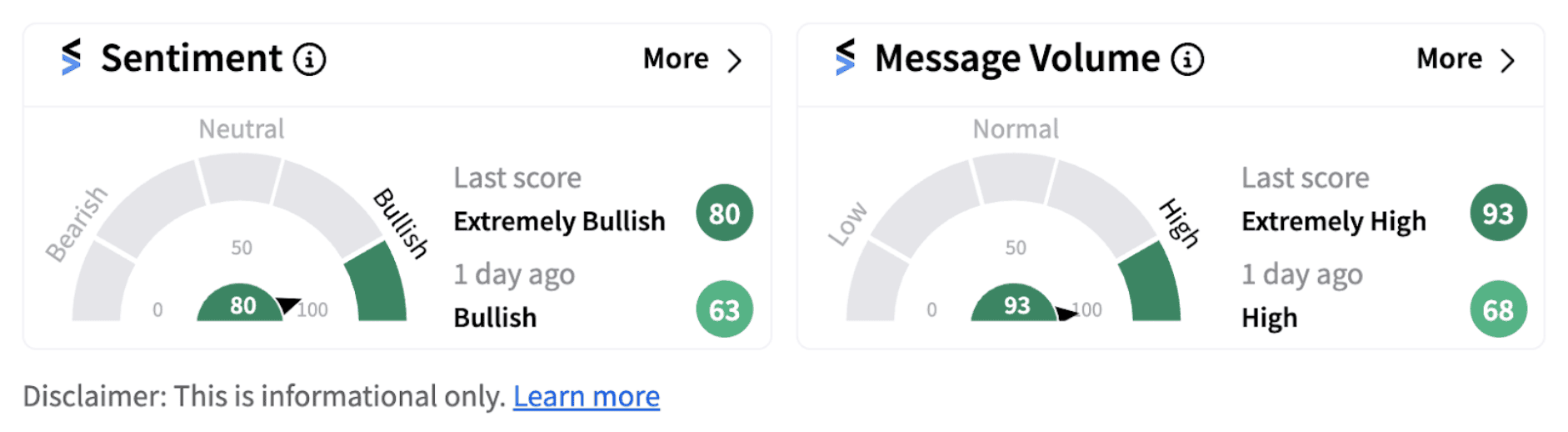
However, Gilead shares have lost 0.5% on a year-to-date basis. Still, bullish followers of the stock are expressing optimism on the latest development.
Also See: McDonald’s Extends $5 Value Meal Till December: Retail Remains ‘Bearish’











/filters:format(webp)https://news.stocktwits-cdn.com/large_Trending_stock_resized_may_jpg_bc23339ae7.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/Rounak_Author_Image_7607005b05.png)
/filters:format(webp)https://news.stocktwits-cdn.com/Getty_Images_2164981884_jpg_df1cf8a926.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/shivani_photo_jpg_dd6e01afa4.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2203138957_jpg_dd735f9905.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2221265139_jpg_2366800575.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/vivekkrishnanphotography_58_jpg_0e45f66a62.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_mohamed_el_erian_jpg_4608eef11b.webp)