Advertisement|Remove ads.
Hims & Hers Stock Slides After FDA Says Eli Lilly’s Weight-Loss Drug No Longer In Shortage, But Retail Shrugs Off Worries
Shares of telehealth company Hims & Hers Health Inc. ($HIMS) fell over 8% pre-market on Thursday following a key update from the federal government on weight-loss drug supply.
Late Wednesday, the U.S. Food and Drug Administration announced that Eli Lilly’s popular weight-loss and diabetes drugs, Mounjaro and Zepbound, are no longer in shortage after a two-year period where supply couldn’t meet skyrocketing demand.
Both medications, which trigger the hormone GLP-1 to help curb appetite, are now available to meet projected national needs, according to the agency.
Investors worry that the update could pose a headwind for telehealth companies like Hims & Hers, which have capitalized on the shortages by offering compounded versions of GLP-1 drugs.
These alternatives, produced by FDA-registered outsourcing facilities, are sold to customers unable to access originals such as Novo Nordisk’s (NVO) Wegovy or Lilly’s Zepbound.
Under U.S. law, telehealth platforms are only allowed to manufacture these copycat drugs when the branded originals are in short supply.
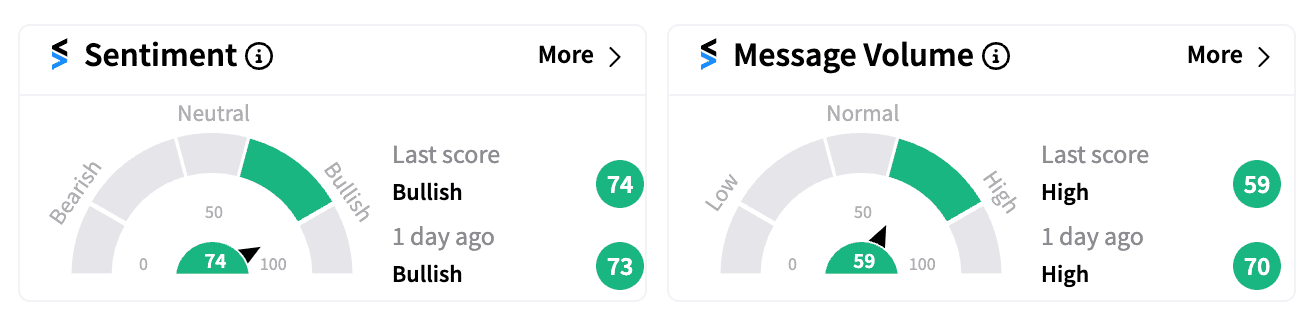
Still, retail sentiment for Hims & Hers remained resilient on Stocktwits, where the ticker held a ‘bullish’ score (74/100) early Thursday.
“It’s going to take a while for the market to realize this is not just a semaglutide company,” wrote one user, while another pointed out that Hims & Hers never sold a compounded version of Tirzepatide (Lilly’s drug).
Citi analysts pointed out that Hims & Hers won’t be directly affected by the FDA’s decision, as it compounds semaglutide, the key ingredient in Novo Nordisk’s rival weight-loss drug, which is still on the FDA’s shortage list.
However, they cautioned that the FDA’s announcement constrains Hims & Hers's future total addressable market and "portend[s] a faster-than-anticipated resolution to shortages."
Hims & Hers is best known for selling treatments like hair-loss products and sexual-health medications like Viagra and Cialis.
The company recently entered the weight-loss space as GLP-1 drugs gained popularity, offering compounded versions with the same active ingredient as Novo Nordisk’s Wegovy and Ozempic.
In May, the company said its weight-loss business is expected to surpass $100 million in revenue by the end of 2025. This projection did not include the revenue potential of GLP-1 drugs, which could further bolster the company’s growth.
Some analysts have noted that Hims & Hers is growing at over 40% annually, even without significant contributions from these drugs.
HIMS stock has nearly doubled in value this year, although Thursday’s pre-market drop reflects the market’s ongoing sensitivity to developments in the GLP-1 space.
Editor's note: This article was updated with Citi's note on the potential impact of the FDA's statement on Hims & Hers' business and addressable market.
Read next: Nikola Stock Thunders Ahead After Impressive Q3 Electric Truck Deliveries, Attracts Retail Spotlight
For updates and corrections, email newsroom@stocktwits.com.













/filters:format(webp)https://news.stocktwits-cdn.com/large_Novo_Nordisk_jpg_96dd19f953.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/vivekkrishnanphotography_58_jpg_0e45f66a62.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2246906030_jpg_5d1c52da00.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/Anushka_Basu_make_me_smile_in_the_picture_b92832aa_af59_4141_aacc_4180d2241ba8_1_2_png_1086e0ed8c.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/Getty_Images_2246745935_jpg_973c37103a.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/shivani_photo_jpg_dd6e01afa4.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2203138957_jpg_dd735f9905.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/Rounak_Author_Image_7607005b05.png)
/filters:format(webp)https://news.stocktwits-cdn.com/large_tim_cook_OG_jpg_08b852f801.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Coinbase_c429427aa1.webp)