Advertisement|Remove ads.
ImmunityBio Stock Tumbles 18% After FDA Issues Refuse To File Letter: But Retail’s Positive

Shares of ImmunityBio, Inc. (IBRX) tumbled 18% on Monday afternoon after the company announced that it received a Refuse to File (RTF) letter from the U.S. Food and Drug Administration (FDA) regarding its bladder cancer treatment application.
The FDA issues refuse-to-file letters if it identifies deficiencies in a company’s application for drug approval.
ImmunityBio received an RTF letter regarding a supplemental biologics license application it filed for the use of ANKTIVA plus Bacillus Calmette-Guerin (BCG) in a specific case scenario of non-muscle invasive bladder cancer (NMIBC).
ImmunityBio said that the application was submitted in March after all key decision makers at the FDA encouraged it to submit the application “as soon as possible” at an in-person meeting in January.
“At this meeting, all key decision makers were specifically asked and unanimously confirmed that ImmunityBio should submit the sBLA as soon as possible based on the data in the single-arm trial,” the company said.
The company also said that it has requested an urgent meeting with the FDA to resolve the inconsistencies between the directives it received in January and the letter it recently received, adding that it was “shocked” by the FDA's “inconsistent response.”
The company also highlighted that the FDA approved the use of ANKTIVA plus BCG last year for another scenario of NMIBC based on the same trial data that it referred to in the recently submitted application.
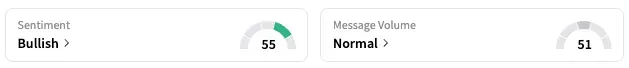
On Stocktwits, retail sentiment around ImmunityBio jumped from ‘bearish’ to ‘bullish’ over the past 24 hours while message volume rose from ‘low’ to ‘normal’ levels.
IBRX stock is down by 19% this year and over 73% over the past 12 months.
Also See: Apple Escalates Epic Games Fight With Appeal – Stock Falls After Price Target Cut
For updates and corrections, email newsroom[at]stocktwits[dot]com.












/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2254648547_jpg_a843db78b6.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/Anushka_Basu_make_me_smile_in_the_picture_b92832aa_af59_4141_aacc_4180d2241ba8_1_2_png_1086e0ed8c.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_1000648682_jpg_6aa61e3574.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2259602028_jpg_5b1a490e64.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2259775985_jpg_a06a1e88c3.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_dogecoin_OG_2_jpg_304df31f25.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_vitalik_buterin_OG_jpg_7ac8ea93fe.webp)