Advertisement|Remove ads.
Moderna Stock Slides After-Hours On CMV Vaccine Failure In Phase 3 Trial — Retail Traders Warn Of ‘Endless’ Cash Burn

- Moderna said its cytomegalovirus (CMV) vaccine, mRNA-1647, failed to prevent infection in women of childbearing age in a large Phase 3 trial, prompting the company to end its congenital CMV program.
- The biotech will continue testing the vaccine in bone marrow transplant patients, where CMV reactivation can cause serious complications, and said no safety concerns were identified.
- Moderna reaffirmed its 2025 guidance and breakeven goal for 2028, noting the vaccine was expected to be cash-flow negative that year.
Moderna Inc. shares fell 4% in after-hours trading on Wednesday after the biotech company said its investigational cytomegalovirus (CMV) vaccine, mRNA-1647, failed to meet its primary efficacy endpoint in a late-stage trial.
Phase 3 Trial Misses Target
The Phase 3 trial was designed to determine if the vaccine can prevent CMV infection among seronegative women aged 16-40. Moderna stated that the vaccine efficacy was substantially below the company's expectations and fell between 6 and 23% depending on the case definition used. The company will not continue the development of mRNA-1647 for the prevention of congenital CMV.
“Today's announcement is disappointing for families and healthcare professionals who have been eagerly awaiting a CMV vaccine to prevent congenital CMV,” said CEO Stephane Bancel in a statement. “We will share full results with the scientific community in hopes that our learnings contribute to the continued pursuit of a CMV vaccine.”
Focus Shifts To Transplant Patients
Moderna will further evaluate mRNA-1647 in a Phase 2 trial for bone marrow transplant recipients, who can experience serious complications when CMV is reactivated. The company said it will investigate the vaccine’s ability to suppress CMV reactivation in this high-risk population.
The company said mRNA-1647 was well-tolerated with no safety concerns identified by the trial’s monitoring board.
No Impact On Financial Outlook
Moderna said the setback is not expected to impact its 2025 financial guidance or its path to breakeven in 2028. The company had expected low revenue for the CMV vaccine in the near term before investments in the market and for the product to be cash-flow negative in 2028.
Stocktwits Users Debate Cash Burn, Oncology Focus After Vaccine Failure
On Stocktwits, retail sentiment for Moderna was ‘bearish’ amid ‘normal’ message volume.
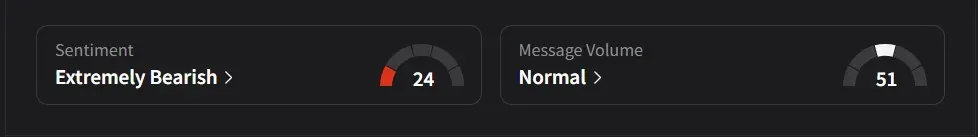
One user suggested the company’s decision to discontinue the CMV program might actually help reduce its cash burn, saying the move could allow Moderna to concentrate more effectively on its oncology pipeline.
Another user expressed concern that the company’s spending remained unsustainable, describing its cash burn as “endless.”
A third bearish user took a harsher view, warning that the stock could fall below $5, after revealing they held two sold naked calls expiring this week.
Moderna’s stock has declined 35% so far in 2025.
For updates and corrections, email newsroom[at]stocktwits[dot]com.












/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2262920033_jpg_f596c67fd3.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/Prabhjote_DP_67623a9828.jpg)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2243565350_jpg_6cd80dbe6d.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/IMG_8805_JPG_6768aaedc3.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/Getty_Images_2227994766_jpg_090ba3c9b6.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/IMG_4530_jpeg_a09abb56e6.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_1463539842_jpg_bcfa58ea0b.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_trump_jpg_fc59d30bbe.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_lucid_stock_jpg_167f2bc3dd.webp)