Advertisement|Remove ads.
Moderna To Reduce R&D Expenses By $1B Through 2027, Focus On 10 New Product Approvals: Retail In Wait-And-Watch Mode
Moderna Inc (MRNA) said on Thursday it expects to reduce its annual research and development expense by approximately $1.1 billion, from an estimated $4.8 billion in 2024 to $3.6-3.8 billion in 2027.
Moreover, the company will expand its commercial portfolio into oncology, rare diseases, and first-in-class non-respiratory vaccines that will potentially yield 10 product approvals over the next three years.
The announcement comes at a time when the firm is witnessing a significant slide in its Covid-19 business. Not surprisingly, shares of Moderna are down over 29% this year.
Investor confidence took a hit when Moderna trimmed its full-year forecast earlier due to very low EU sales in 2024, potential revenue deferrals for certain international sales into 2025, and an increasingly competitive environment for respiratory vaccines in the U.S.
The firm stated on Thursday it has discontinued five programs in its pipeline that include Endemic HCoV, RSV infants, KRAS antigen-specific therapy, among others.
The biotech major now expects 2025 revenue in the range of $2.5 billion to $3.5 billion versus a Wall Street estimate of $3.94 billion. For 2026-2028, the company expects a compounded annual growth rate of over 25% or more, driven by new product launches.
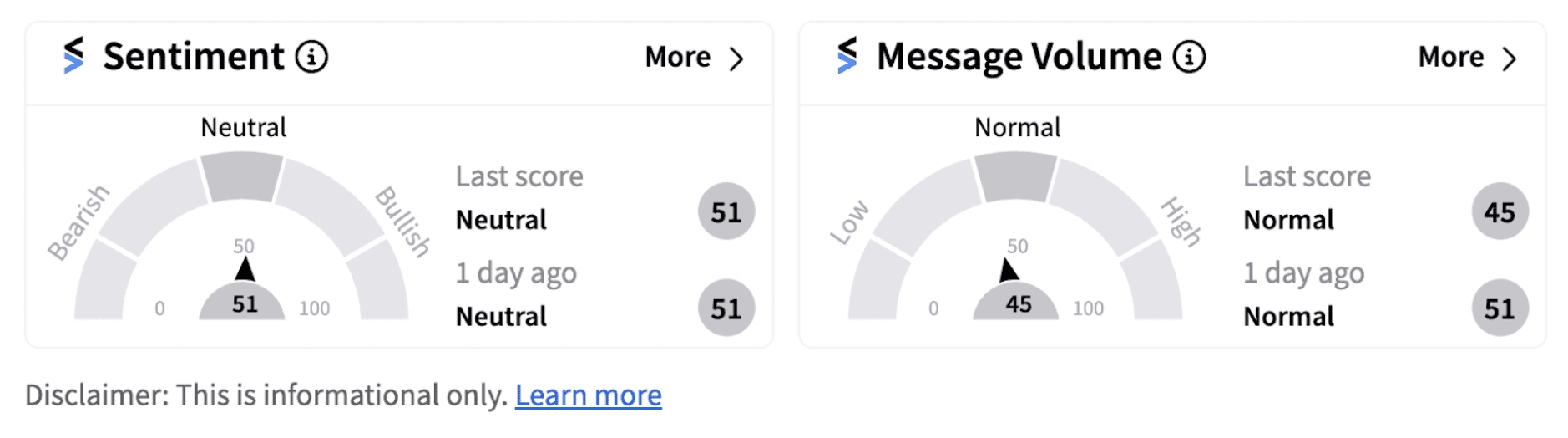
Following the announcement, shares of Moderna sank nearly 7% in Thursday’s pre-market session as of 6:54 a.m. ET. Retail sentiment on Stocktwits continued to remain in the ‘neutral’ territory as investors digested the projections.
Moderna is also reducing its expected research and development investment for 2025-2028 by approximately 20%, from $20 billion for the period to $16 billion.
Stéphane Bancel, CEO of Moderna said that the firm now has five respiratory vaccines with positive Phase 3 results and expects to submit three for approval this year. “In addition, we have five non-respiratory products in pivotal studies across cancer, rare diseases and latent vaccines with potential for approval by 2027,” Bancel stated.
Moderna also announced that its RSV vaccine, mRESVIA (mRNA-1345), has been approved for adults aged 60 years and older in the U.S. and the EU. mRNA-1345 is in an ongoing Phase 3 study designed to test immunogenicity and safety in high-risk adults belonging to the 18 to 59 years age category.
Bearish followers of the ticker on Stocktwits appear to be skeptical about the firm’s latest announcement. One user named ‘Kgibson71’ believes the stock may even fall to the 20s sometime next year.










/filters:format(webp)https://news.stocktwits-cdn.com/large_App_Lovin_jpg_42d40549b1.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/Aashika_Suresh_Profile_Picture_jpg_2acd6f446c.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2256305566_jpg_26cd17b56a.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/jaiveer_jpg_280ad67f36.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_natural_gas_plant_resized_jpg_e43db2dc7b.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/vivekkrishnanphotography_58_jpg_0e45f66a62.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/large_cpi_inflation_generic_resized_7abe1d339c.jpg)
/filters:format(webp)https://news.stocktwits-cdn.com/large_kyndryl_stock_resized_jpg_805a2b8f99.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2206289958_jpg_e8a549e3a1.webp)