Advertisement|Remove ads.
Novartis Stock Rises Premarket After FDA Approves First Gene Therapy For Teens And Adults With Muscle-Weakening Disorder

- FDA cleared Itvisma as the first gene-replacement therapy available to people 2 and older with the rare muscle-weakening condition.
- Phase III data showed improved or stabilized motor function with a single intrathecal dose.
- Novartis expanded its gene therapy footprint with exclusive global rights to key AAV9 and recombinant AAV delivery platforms.
Novartis shares rose over 1% in premarket trading on Tuesday after the company received U.S. regulatory approval for Itvisma, the first gene replacement therapy cleared for use in children two years and older, teenagers, and adults living with spinal muscular atrophy (SMA), a rare genetic condition that progressively weakens muscles used for movement, breathing, and swallowing.
FDA Greenlights A One-Time Gene Therapy
The FDA authorized Itvisma as a single-dose treatment designed to replace the missing SMN1 gene that causes SMA. Novartis said the therapy can improve motor function and may reduce the need for long-term, chronic treatment required with other options.
The approval makes Itvisma the only gene therapy available for older children, teens, and adults with SMA, greatly expanding eligibility beyond infants and toddlers who historically had the most access to gene-based treatments.
This approval is a “game-changing advance,” said John W. Day of Stanford University, calling it a significant milestone not only for SMA care but for the broader field of neurological genetic medicine.
Strong Clinical Results
The decision was supported by data from Phase III trials (STEER and STRENGTH), which showed improved or stabilized motor abilities over 52 weeks, marking an outcome not typically seen in the natural course of the disease. The safety profile was consistent across both studies, with common side effects including respiratory infections, fever and cold symptoms.
Cure SMA, a leading advocacy group, said the therapy’s single-dose design offers potential “greater independence and freedom in daily life” for patients who have long relied on continuous treatment.
SMA: A Rare, Progressive Condition
SMA is due to the absence of or a defective SMN1 gene. The SMN1 gene provides instructions for the body to support muscle control and prevent nerve-cell deterioration. If left untreated, the body loses motor neurons, resulting in severe, sometimes fatal, muscle weakness. Nearly 9,000 people in the U.S. are living with the condition.
Rollout Begins In December
Itvisma will be available in the U.S. beginning in December, and Novartis said patient-support services will help eligible individuals navigate insurance and financial assistance. The company also underscored the technology foundation behind the therapy, noting that it holds exclusive worldwide rights with Nationwide Children’s Hospital for AAV9 gene-replacement delivery in SMA, as well as a separate global license from REGENXBIO covering the use of recombinant AAV vectors for in vivo SMA gene therapy.
Stocktwits Mood Stays Bearish
On Stocktwits, retail sentiment for Novartis was ‘bearish’ amid ‘low’ message volume.
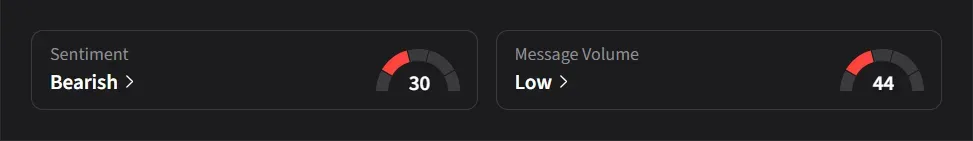
Novartis’ stock has risen 33% so far in 2025.
For updates and corrections, email newsroom[at]stocktwits[dot]com.










/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2263898051_jpg_9e75888009.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/IMG_8805_JPG_6768aaedc3.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2241292402_jpg_9661b0c852.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/unnamed_jpg_9dff551b50.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/large_stock_rising_resized_f17852d7aa.jpg)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/large_adam_smigielski_K5m_Pt_O_Nmp_HM_unsplash_f365ee93f4.jpg)
/filters:format(webp)https://news.stocktwits-cdn.com/jaiveer_jpg_280ad67f36.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2058827032_jpg_5505a2a083.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/Getty_Images_526218674_jpg_7b7468812b.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/Aashika_Suresh_Profile_Picture_jpg_2acd6f446c.webp)