Advertisement|Remove ads.
Novavax Tops Weekly Retail Buzz Spike Amid FDA Trial Drama, COVID-19 Vaccine Approval Hopes
Novavax, Inc. (NVAX) shares jumped nearly 12.6% over the past week, leading to a surge in retail investor chatter following renewed hopes for a regulatory nod for its COVID-19 vaccine, which has been available since 2022 only under emergency use authorization.
Last week, Novavax said it believes its Biologics License Application (BLA) for its COVID-19 vaccine, Nuvaxovid, is approvable based on ongoing conversations with regulators.
“We believe that our Biologics License Application is approvable based on conversations with the U.S. Food and Drug Administration, as of our Prescription Drug User Fee Act - PDUFA - date of April 1 and through today,” the company said on Wednesday.
“We have recently received formal communication from the FDA in the form of an information request for a postmarketing commitment to generate additional clinical data.”
Novavax said it looks forward to addressing the request and moving toward approval as soon as possible.
BofA Securities noted Novavax shares rose after the company said the requested data would be gathered post-approval, helping ease some investor concerns.
However, BofA reiterated a 'Neutral' rating and $10 price target, citing a need for more clarity around the company's commercial plans.
The optimism was tempered by a Wall Street Journal report on Friday, which said federal regulators had asked Novavax to conduct another randomized clinical trial of its COVID-19 vaccine — a move that could prove financially burdensome.
The report noted that appointees under Health and Human Services Secretary Robert F. Kennedy Jr. intervened, requesting further proof of efficacy.
Despite the mixed news flow, Stocktwits data showed sentiment for Novavax turned 'bullish' by the end of the week, improving from 'neutral' levels earlier.
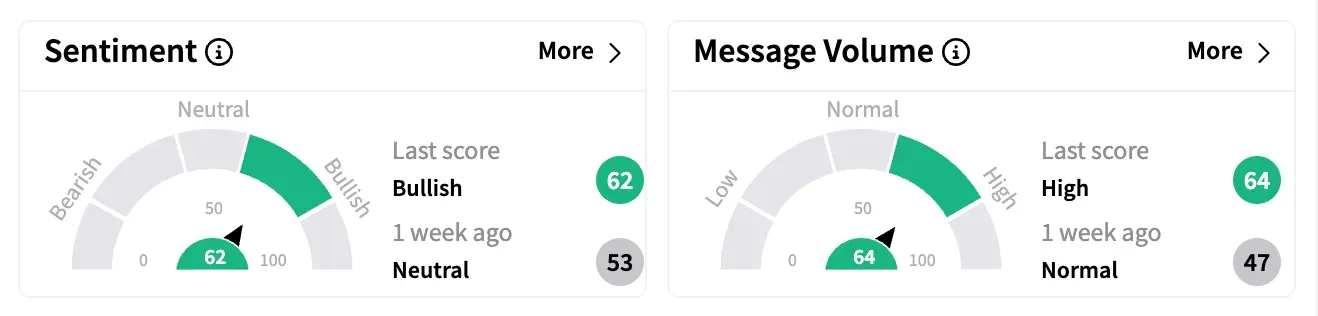
Weekly message volume for NVAX on the platform soared 1,758%, the highest among all tickers.
"I believe all of this is a coordinated media campaign designed to shake out weak hands. Everything points to the fact that once the BLA is approved, the trend for NVAX will reverse to the upside," wrote one bullish user.
"Overall, the PMC framework supports a dynamic and sustainable position for Novavax in the evolving COVID-19 vaccine market," said another.
Nuvaxovid, Novavax's protein-based COVID-19 shot, has been under renewed scrutiny since Kennedy Jr. publicly questioned the vaccine's efficacy earlier this month.
Unlike mRNA-based vaccines from Pfizer-BioNTech and Moderna, Nuvaxovid uses a traditional protein subunit platform.
Novavax shares are down over 17% year-to-date but have climbed nearly 63% over the past 12 months.
For updates and corrections, email newsroom[at]stocktwits[dot]com.












/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/large_microstrategy_michael_saylor_resized_9fd19e69ec.jpg)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/Prabhjote_DP_67623a9828.jpg)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2262651778_jpg_54075aa1d9.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/IMG_9209_1_d9c1acde92.jpeg)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/large_ibm_signage_mwc_resized_28f91e1a63.jpg)
/filters:format(webp)https://news.stocktwits-cdn.com/large_circle_stablecoins_original_jpg_b238d12be8.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/large_US_stocks_3e2253bcca.jpg)
/filters:format(webp)https://news.stocktwits-cdn.com/Revised_Profile_JPG_0e0afdf5e2.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2209881066_jpg_ebc4b9b217.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/IMG_4530_jpeg_a09abb56e6.webp)