Advertisement|Remove ads.
Omeros Corp Sells Immune-System Drug To Novo Nordisk In $2.1B Deal — Retail Shifts Focus To Narsoplimab FDA Call

- Omeros received $240 million upfront, with total deal value reaching up to $2.1 billion.
- The company retired its senior loan and expects the remaining cash to extend its runway.
- Traders say the FDA decision on Narsoplimab is now the key market catalyst.
Omeros Corporation (OMER) said on Monday it has completed its asset sale and licensing agreement with Novo Nordisk for Zaltenibart, an experimental drug being developed for rare immune-related blood and kidney disorders. The transaction includes up to $2.1 billion in potential payments.
OMER stock traded in the green at the time of writing.
Terms Of The Deal
Omeros received $240 million upfront at closing and could earn up to $340 million in early milestones. The agreement grants Novo Nordisk global rights to Zaltenibart and its intellectual property, with total potential value reaching $2.1 billion through future development and commercial milestones. Omeros will also receive tiered royalties on net sales.
Immune Drug Targets Severe Rare Diseases
Zaltenibart, formerly OMS906, is a clinical-stage monoclonal antibody that blocks MASP-3, the immune trigger that switches on the alternative complement pathway. The drug is designed to calm an overactive immune response while preserving the body’s main infection-fighting mechanisms. Potential applications include paroxysmal nocturnal hemoglobinuria and several complement-driven kidney disorders. Novo Nordisk said the acquisition strengthens its position in rare immunological diseases.
Upfront Proceeds Slash Debt And Extend Cash Runway
Omeros used part of the upfront payment to fully repay its $67.1 million senior secured term loan, including premiums and interest, resulting in the release of all related covenants. The company expects remaining proceeds to cover repayment of its $17.1 million 2026 Convertible Notes and fund more than a year of operations, including preparations for a potential U.S. launch of its MASP-2 inhibitor Narsoplimab. The company continues to advance additional complement-system candidates, including OMS1029 and OMS527.
Stocktwits Users Warn Of FDA-Driven Swings
On Stocktwits, Omeros drew ‘bullish’ retail sentiment with ‘normal’ message volume, while Novo Nordisk saw ‘bearish’ sentiment on ‘low’ volume.
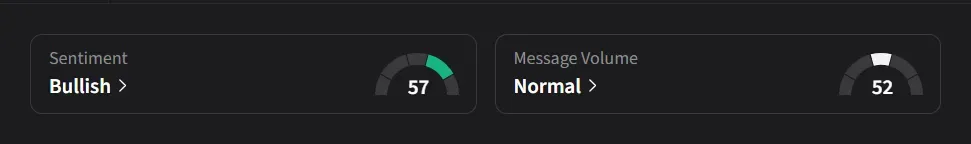
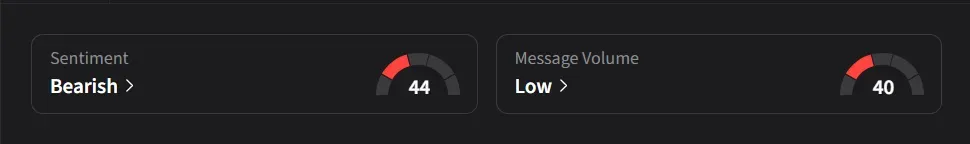
One user noted that the announcement appeared mostly priced in around $10 and said the stock now hinges on a potential FDA approval for Zaltenibart due Dec.26, calling it a "pure binary event" that could swing shares 30%–50% either way over the next few weeks.
Another user said, “This deal was known for a long time already. What did you expect? Narsoplimabs FDA approval this month is the moneyball.”
Omeros’ stock has declined nearly 2% so far in 2025, while Novo Nordisk’s U.S.-listed stock fell about 42% over the same period.
For updates and corrections, email newsroom[at]stocktwits[dot]com.











/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/large_conocophillips_resized_98da51d9b9.jpg)
/filters:format(webp)https://news.stocktwits-cdn.com/Aashika_Suresh_Profile_Picture_jpg_2acd6f446c.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2239226376_jpg_c72fd10c8b.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/unnamed_jpg_9dff551b50.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/large_sealsq_stock_market_representative_resized_b05435011f.jpg)
/filters:format(webp)https://news.stocktwits-cdn.com/large_App_Lovin_jpg_42d40549b1.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/jaiveer_jpg_280ad67f36.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_US_economy_representative_image_jpg_88c3aa4736.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2229385023_jpg_648b095662.webp)