Advertisement|Remove ads.
Replimune Stock Plunged 42% Today – Here’s Why

Replimune Group, Inc. (REPL) announced on Thursday that a path forward for approval of its lead candidate for the treatment of advanced melanoma has not been determined, despite a meeting with the U.S. Food and Drug Administration. Shares of the company slumped 42% at the time of writing.
The FDA issued a complete response letter regarding the application for RP1 in combination with Nivolumab for the treatment of advanced melanoma in late July. The agency said in the letter that it is unable to approve the application in its present form and indicated that the trial conducted by the company for the drug is not considered to be an adequate and well-controlled clinical investigation that provides substantial evidence of effectiveness.
The company subsequently requested a meeting with the agency to push for approval of RP1. “We remain steadfastly committed to patient access while we work with the FDA to secure regulatory approval for RP1, however, without accelerated approval based on the current application, continuation of the RP1 program in advanced melanoma, including the phase 3 confirmatory trial, will not be viable,” CEO Sushil Patel said earlier this month.
However, on Thursday, the company stated that it had completed a meeting with the FDA on September 16 regarding the letter pertaining to the application for approval of RP1. The company stated that it is evaluating the feedback from the FDA to determine its next steps, and the path forward under the accelerated approval pathway has not yet been determined.
“We remain committed to working with the FDA to determine an expeditious path forward for RP1,” Patel reiterated on Thursday.
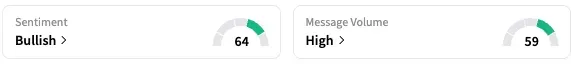
RP1 is Replimune’s lead product candidate. On Stocktwits, retail sentiment around REPL stock jumped from ‘neutral’ to ‘bullish’ territory over the past 24 hours, while message volume rose from ‘low’ to ‘high’ levels.
A Stocktwits user opined that a strategic review announcement is due in the coming months.
A more optimistic user, however, said that RP1 remains “a therapy to watch” for melanoma patients.
REPL stock is down by about 73% this year and by about 70% over the past 12 months.
Read also: GE Healthcare Reportedly Eyes Selling Stake In China Unit – More Details Inside
For updates and corrections, email newsroom[at]stocktwits[dot]com.













/filters:format(webp)https://news.stocktwits-cdn.com/large_Rocketlab_resized_jpg_92c1a02a7f.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/unnamed_jpg_9dff551b50.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2201668075_jpg_cae68c6d02.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/jaiveer_jpg_280ad67f36.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/Getty_Images_2197860201_jpg_c4f2083335.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/large_stock_rising_resized_f17852d7aa.jpg)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2250993932_jpg_77e7b26c88.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Block_Inc_logo_displayed_on_smartphone_screen_6065fd32bb.webp)