Advertisement|Remove ads.
This Smallcap Pharma Stock Is Gaining Retail Buzz Ahead Of A Key FDA Ruling

- Tradipitant is a neurokinin-1 receptor antagonist licensed by Vanda from Eli Lilly and Company.
- The drug is also being developed to treat gastroparesis.
- Last month, Vanda reported positive topline results from a placebo-controlled study showing reduced nausea and vomiting linked to the GLP-1 drug Wegovy in overweight and obese adults.
Vanda Pharmaceuticals (VNDA) shares climbed nearly 8% in premarket trading on Friday, drawing strong retail interest as investors positioned ahead of an upcoming U.S. Food and Drug Administration (FDA) decision on Tradipitant, the company’s motion-sickness treatment aimed at preventing vomiting.
VNDA was among the top trending stocks at the time of writing and is on track to open at its highest level since February 2023.
All Eyes On Pending FDA Nod
Tradipitant is a neurokinin-1 receptor antagonist licensed by Vanda from Eli Lilly and Company (LLY). The drug is being developed for gastroparesis and GLP-1–related nausea and vomiting, and is also under FDA review for motion sickness, with a PDUFA date of December 30, 2025. A PDUFA date is the FDA's target date to make a decision on a drug application. The FDA also provided feedback on the proposed labelling.
Last month, Vanda reported positive topline results from a randomized, placebo-controlled study evaluating Tradipitant for preventing nausea and vomiting caused by the GLP-1 drug Wegovy in overweight and obese adults.
The trial met its primary endpoint, demonstrating a 50% reduction in vomiting compared with placebo. Tradipitant also demonstrated a favorable safety profile with no new signals. Vanda plans to advance the program toward Phase III in 2026.
How Did Stocktwits Users React?
Retail sentiment for VNDA on Stocktwits remained ‘bullish’ over the past 24 hours.
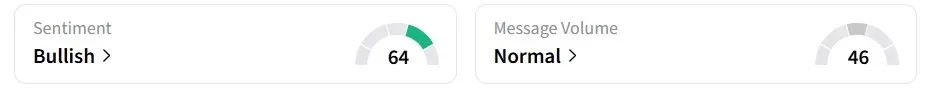
Users expect the FDA to approve the drug, with one watcher stating that once a drug application is resubmitted and assigned a new PDUFA date, approval becomes the most likely outcome.
Vanda had filed a New Drug Application (NDA) with the FDA in September 2023, but a year later, the agency issued a Complete Response Letter, stating that the submission did not yet demonstrate sufficient evidence of efficacy.
Year-to-date, VNDA stock has seen strong buying interest, gaining over 40%.
For updates and corrections, email newsroom[at]stocktwits[dot]com











/filters:format(webp)https://news.stocktwits-cdn.com/Getty_Images_2233055049_jpg_0a316df698.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/jaiveer_jpg_280ad67f36.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2228864541_jpg_d94770c5a8.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/vivekkrishnanphotography_58_jpg_0e45f66a62.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Sandisk_jpg_920fcc1fc3.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/Aashika_Suresh_Profile_Picture_jpg_2acd6f446c.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/large_novavax_vaccine_resized_455cef63e9.jpg)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/unnamed_jpg_9dff551b50.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2156649816_jpg_bde9d5ac58.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2230529087_jpg_ce4582941c.webp)