Advertisement|Remove ads.
CMPS Stock Climbs 5% In Premarket After FDA Clears PTSD Trial Path
- FDA has accepted the company's Investigational New Drug application for COMP360.
- The latest update clears the way for a late-stage Phase 2b/3 clinical trial.
- The company said prior Phase 2 data showed the treatment was generally well tolerated.
Compass Pathways (CMPS), on Wednesday, said the U.S. Food and Drug Administration (FDA) has accepted its Investigational New Drug application for COMP360, a drug to treat post-traumatic stress disorder (PTSD). The latest update clears the way for a late-stage Phase 2b/3 clinical trial.
The study will evaluate the efficacy, safety, and tolerability of COMP360, with a blinded 12-week phase followed by a long-term open-label extension. The company said prior Phase 2 data showed the treatment was generally well tolerated and delivered rapid, durable symptom improvement.
Compass also highlighted ongoing FDA discussions around a potential rolling NDA submission for treatment-resistant depression, with additional Phase 3 data expected in 2026.
“PTSD is one of the most challenging mental health conditions, with approximately 13 million adults in the U.S. living with persistent symptoms and limited treatment options. We believe COMP360 has the potential to transform the treatment landscape for PTSD and bring hope to those who need it most,” said Guy Goodwin, Chief Medical Officer at Compass Pathways.
CMPS stock was up over 5% in pre-market trading on Wednesday.
Earlier this week, Compass Pathways entered its seventh strategic collaboration, partnering with Radial Health, a national network of interventional psychiatry practices.
The agreement is aimed at developing scalable care models for COMP360 psilocybin treatment, if approved, across a wide range of U.S. mental health care settings.
How Did Stocktwits Users React?
Retail sentiment for CMPS on Stocktwits turned 'bullish' from 'bearish' a day earlier.
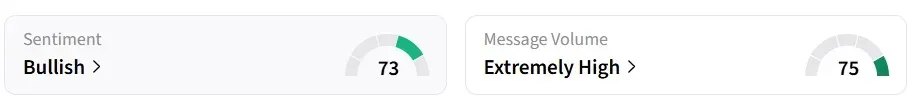
One user expects the stock to climb to $15 over the next few days.
The stock has gained nearly 60% this year.
Read also: Why Did First Solar Stock Fall 5% In Premarket Today?
For updates and corrections, email newsroom[at]stocktwits[dot]com.











/filters:format(webp)https://news.stocktwits-cdn.com/large_Tether_2376a55503.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/Anushka_Basu_make_me_smile_in_the_picture_b92832aa_af59_4141_aacc_4180d2241ba8_1_2_png_1086e0ed8c.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_robert_kiyosaki_d28a01cb4b.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Bitcoin_and_Ethereum_2b4356b70a.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_976546456_jpg_42ddd4a81d.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2259655311_jpg_20124bbeb9.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Donald_Trump_451371e34e.webp)