Advertisement|Remove ads.
Why Did AQST Stock Jump 35% Today?
- The FDA’s concerns were limited to packaging, labeling, and administration issues, the company said.
- Aquestive has already modified the pouch design, updated instructions for use, and revised labelling.
- The firm is also targeting global expansion plans for Anaphylm after initiating regulatory discussions in Canada, Europe, and the U.K last year.
Shares of Aquestive Therapeutics (AQST) surged more than 35% on Monday morning, after receiving a Complete Response Letter (CRL) from the U.S. Food and Drug Administration (FDA) for its New Drug Application (NDA) seeking approval of Anaphylm, a sublingual film to treat severe Type I allergic reactions.
A CRL indicates that while a drug has not been approved in its current form, it allows the sponsor to address the issues raised by the FDA and seek re-approval.
FDA’s Concerns
The FDA’s concerns were limited to packaging, labeling, and administration issues identified in a human factors validation study. The FDA noted that some users had difficulty opening the pouch and correctly placing the film, which could pose safety risks during emergency situations. The agency did not raise questions about the drug’s comparability data or manufacturing processes, the company said.
To address the feedback, Aquestive has modified the pouch design, updated instructions for use, and revised labeling. The company plans to conduct a new human factors study alongside a single pharmacokinetics (PK) study requested by the FDA. No additional trials are required.
Based on the initial review, Aquestive expects to resubmit the application as early as the third quarter of 2026 and will seek a rapid review.
Europe Update
Aquestive is also targeting global expansion plans for Anaphylm after initiating regulatory discussions in Canada, Europe, and the U.K. in 2025. The European Medicines Agency indicated that no additional clinical trials are required, with submissions in Europe and Canada expected in the second half of 2026. The company also anticipates feedback from the U.K.’s MHRA in the first quarter of 2026.
How Did Stocktwits Users React?
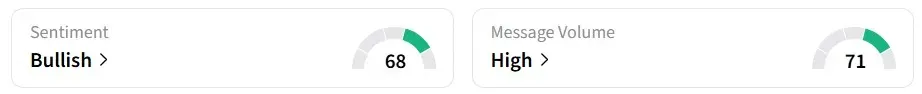
Retail sentiment on Stocktwits turned ‘bullish’ from ‘neutral’ a session earlier, amid ‘high’ message volumes.
One user brushed off concerns about the CRL, noting there were no key issues.
While the stock has gained nearly 35% over the past year, its year-to-date losses are around 40%.
For updates and corrections, email newsroom[at]stocktwits[dot]com.










/filters:format(webp)https://news.stocktwits-cdn.com/Getty_Images_2231423801_jpg_f64bcdbb33.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/unnamed_jpg_9dff551b50.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2242062032_jpg_b5e44cfa75.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/large_Trade_desk_logo_resized_c0229eb2ab.jpg)
/filters:format(webp)https://news.stocktwits-cdn.com/jaiveer_jpg_280ad67f36.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2230137825_jpg_d14459f501.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2217651250_jpg_908ef236f4.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2194622714_jpg_c18475d557.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/Aashika_Suresh_Profile_Picture_jpg_2acd6f446c.webp)