Advertisement|Remove ads.
Why Personalis Stock Is Rising: Medicare Covers Lung Cancer MRD Test
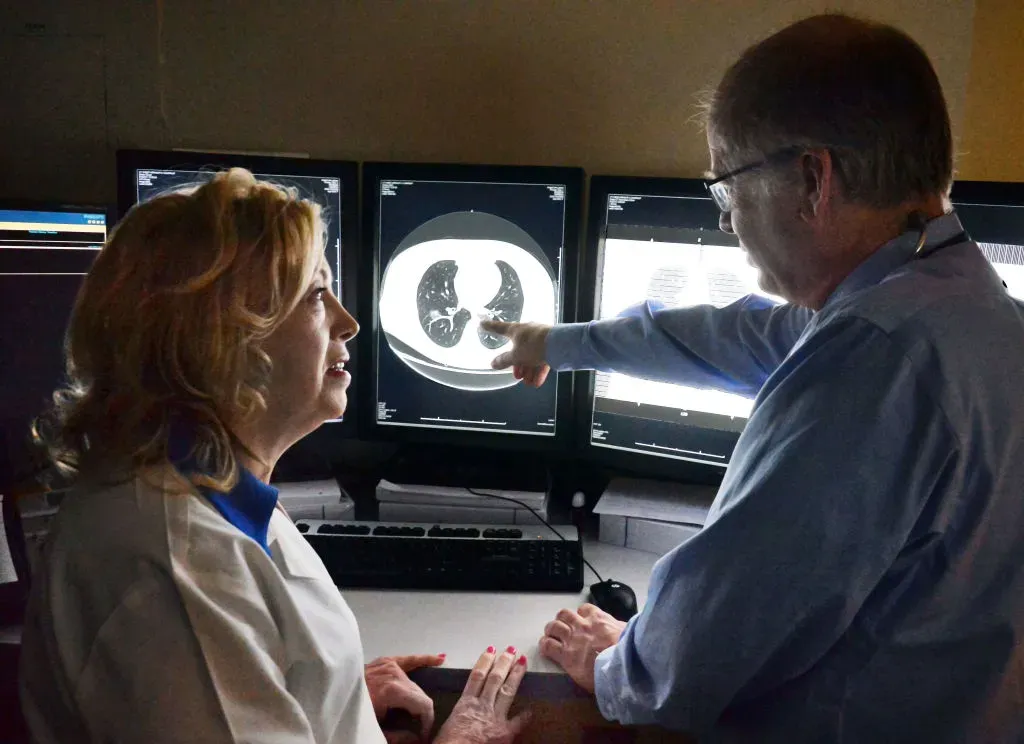
- The company said the decision expands coverage beyond its earlier breast cancer indication.
- NeXT Personal uses whole-genome sequencing and advanced noise-suppression processes to identify tiny amounts of circulating tumor DNA (ctDNA) in a patient’s blood.
- The Medicare decision is backed by data generated through a long-term collaboration with the TRACERx consortium.
Personalis, Inc. (PSNL) announced on Tuesday that Medicare will now reimburse its NeXT Personal molecular residual disease (MRD) test for use in monitoring patients with early- to mid-stage non-small cell lung cancer (NSCLC).
The company stated the decision expands coverage beyond its earlier breast cancer indication, giving broader access to the assay for Medicare beneficiaries.
Why Lung Cancer Coverage Matters
Under the new determination, Medicare will pay for NeXT Personal when used to track cancer recurrence and residual disease in patients with Stage I through Stage III NSCLC.
Lung cancer remains the deadliest cancer in the U.S., with hundreds of thousands of Americans diagnosed each year and a high risk of recurrence following initial treatment.
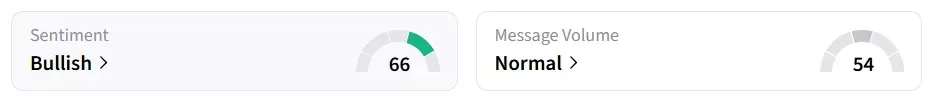
Following the announcement, Personalis’ stock traded over 16% higher in Tuesday’s premarket. On Stocktwits, retail sentiment around the stock jumped to ‘bullish’ from ‘neutral’ territory the previous day amid ‘normal’ message volume levels.
How The NeXt Test Works
NeXT Personal uses whole-genome sequencing and advanced noise-suppression processes to identify tiny amounts of circulating tumor DNA (ctDNA) in a patient’s blood. By tracking up to about 1,800 unique tumor mutations, clinicians can detect signs of residual or returning cancer earlier than with traditional imaging.
"For the first time, Medicare beneficiaries battling lung cancer will have access to ultrasensitive MRD testing, providing the deep insights needed to manage their care with greater confidence.”
-Chris Hall, President and CEO, Personalis
The Medicare decision is supported by data from a long-term collaboration with the TRACERx consortium, which recently published results in Cell, validating the test’s performance in lung cancer surveillance.
For updates and corrections, email newsroom[at]stocktwits[dot]com.











/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2207049226_jpg_7f1e685123.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/jaiveer_jpg_280ad67f36.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2218181377_jpg_f2dccc3db9.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/Aashika_Suresh_Profile_Picture_jpg_2acd6f446c.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2245017747_jpg_f783731632.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/unnamed_jpg_9dff551b50.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_China_i_Phone_jpg_bcedab655a.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/large_American_Airlines_Getty_4d3d704837.jpg)
/filters:format(webp)https://news.stocktwits-cdn.com/vivekkrishnanphotography_58_jpg_0e45f66a62.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_sabre_resized_jpg_fa5aa35db6.webp)