Advertisement|Remove ads.
Alembic Pharma Jumps 12% On USFDA Nod For Cancer Drug; SEBI RA Spots Bullish Breakout

Alembic Pharmaceuticals jumped more than 12% after receiving approval from the U.S. Food and Drug Administration (USFDA) for its generic version of Doxorubicin Hydrochloride Liposome injection.
The drug, used in cancer treatment, falls under a high-value, competitive segment in the U.S. pharmaceutical market.
The clearance is another step in Alembic’s efforts to expand its U.S. generics portfolio.
SEBI-registered research analyst Sunil Kotak said the daily chart for Alembic Pharma reflected a bullish setup.
He noted that both price action and volume are positive, with a breakout visible on the charts.
According to Kotak, the Relative Strength Index (RSI) has crossed above 60 on both daily and weekly charts, signalling positive momentum.
He identified a strong demand zone between ₹1,040 and ₹1,060, and a supply zone between ₹1,160 and ₹1,180.
“The charts are bullish,” Kotak said.
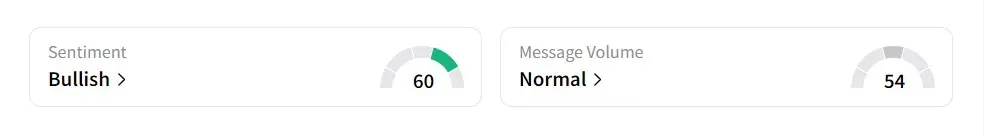
On Stocktwits, retail sentiment was ‘bullish’ amid ‘normal’ message volume.
The stock has risen 3.6% so far in 2025.
For updates and corrections, email newsroom[at]stocktwits[dot]com.












/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2228864541_jpg_d94770c5a8.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/vivekkrishnanphotography_58_jpg_0e45f66a62.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Sandisk_jpg_920fcc1fc3.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/Aashika_Suresh_Profile_Picture_jpg_2acd6f446c.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/large_novavax_vaccine_resized_455cef63e9.jpg)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/unnamed_jpg_9dff551b50.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2156649816_jpg_bde9d5ac58.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2230529087_jpg_ce4582941c.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Meta_jpg_0f17dacb20.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/Rounak_Author_Image_7607005b05.png)