Advertisement|Remove ads.
Eli Lilly Sues Four Telehealth Companies Selling Copies Of Its Weight-Loss Drug, Says Report: But Retail Stays Optimistic

Eli Lilly and Company (LLY) is suing four telehealth companies selling compounded versions of its weight loss drug Zepbound and its diabetes treatment Mounjaro, CNBC reported on Wednesday.
The pharmaceutical giant filed lawsuits against Mochi Health, Fella Health, Willow Health, and Henry Med on Wednesday, alleging that they deceived consumers about “untested, unapproved drugs,” the report said.
Compounders are allowed to produce copies of obesity drugs only when they are in short supply. Mass compounding of tirzepatide, the active ingredient in Mounjaro and Zepbound, was supposed to stop last month after the Food and Drug Administration (FDA) declared that the shortage of the drugs, which started in late 2022, had been resolved.
Lilly alleged that the companies are mass-marketing slightly different versions of its drugs in order to skirt the FDA’s oversight. Some of the sites are selling formulations of the drugs that haven’t been studied, such as oral tablets and drops, it added.
In its lawsuit against Mochi, Lilly claimed that its CEO, Myra Ahmad, is not a licensed physician and that Mochi and its “unlicensed owners exercise undue influence and control over, among other things, the prescribing decisions of physicians,” CNBC reported.
Lilly is seeking to stop the sites from marketing or selling tirzepatide in all four cases.
Earlier this month, Lilly also sued Strive Pharmacy LLC and Empower Clinic Services LLC, alleging they were selling unapproved drugs and marketing them as personalized versions of Lilly’s drugs.
On Stocktwits, retail sentiment around Eli Lilly fell marginally over the past 24 hours but remained within the ‘extremely bullish’ territory, while message volume remained at ‘extremely high’ levels.
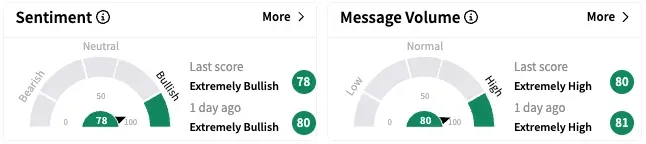
LLY stock is up by over 6% so far this year and by nearly 11% over the past 12 months.
For updates and corrections, email newsroom[at]stocktwits[dot]com












/filters:format(webp)https://news.stocktwits-cdn.com/Getty_Images_1485519874_1_jpg_82b28a6517.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/shivani_photo_jpg_dd6e01afa4.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_rare_earth_july_d867df7d47.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/vivekkrishnanphotography_58_jpg_0e45f66a62.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2203138957_jpg_dd735f9905.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/Rounak_Author_Image_7607005b05.png)
/filters:format(webp)https://news.stocktwits-cdn.com/Getty_Images_2250240969_jpg_dd9be8c5ea.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Intuit_resized_jpg_913ef93c15.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_vir_biotech_jpg_f43ff73654.webp)