Advertisement|Remove ads.
Glucotrack Announces Positive Results From Its Implantable Blood Glucose Monitor Study: Retail Sentiment Hits 3-Month High

Shares of Glucotrack, Inc. (GCTK) traded 32% higher on Wednesday afternoon after the company announced that its first-in-human clinical study for its investigational continuous blood glucose monitor (CBGM) yielded positive results.
The medical device company’s CBGM demonstrated excellent accuracy, a 99% data capture rate, and no procedure or device-related serious adverse events, the company said.
Glucotrack’s CBGM is a long-term, implantable system that continually measures glucose from blood rather than interstitial fluid with a sensor designed for a 3-year life and no on-body wearable component.
It aims to eliminate the lag time associated with traditional continuous glucose monitoring systems and is connected to subcutaneous electronics that communicate with a mobile application.
The in-human study for the CBGM was conducted between Dec. 13, 2024, and Jan. 31, 2025, and included ten participants with either Type 1 or Type 2 diabetes who were on intensive insulin therapy.
The insertion and removal procedures were performed by interventional cardiologists.
Following the device placement, each participant underwent inpatient observation for 4 days. During this period, frequent blood sampling and glucose tolerance tests were conducted to assess device performance.
The system remained in place throughout the study period and was removed successfully at study completion, with patients then followed for 7 days post-removal.
Glucotrack now expects to initiate a long-term early feasibility study in Q3 2025, which will evaluate safety and performance of the monitor over an extended period.
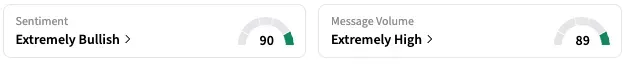
On Stocktwits, retail sentiment around GCTK soared from ‘bearish’ to ‘extremely bullish’ territory over the past 24 hours to its highest levels in 3 months while message volume rose from ‘low’ to ‘extremely high’ levels.
A Stocktwits user expressed optimism for Glucotrack being approached by big pharmaceutical companies for either purchase or partnerships following the news.
GCTK stock is down by about 98% this year and has erased nearly all of its value over the past 12 months.
Read Next: Sarepta Stock Slumps After FDA Opens Probe Into Patient Death, Analyst Sounds Alarm
For updates and corrections, email newsroom[at]stocktwits[dot]com.










/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_1549425533_jpg_483afd6d69.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/unnamed_jpg_9dff551b50.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/large_stock_down_resized_901c19c371.jpg)
/filters:format(webp)https://news.stocktwits-cdn.com/Getty_Images_2235778544_jpg_2b7ceca102.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/Aashika_Suresh_Profile_Picture_jpg_2acd6f446c.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/large_hawaiian_electric_resized_4b766fd741.jpg)
/filters:format(webp)https://news.stocktwits-cdn.com/jaiveer_jpg_280ad67f36.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/large_sealsq_stock_market_representative_resized_b05435011f.jpg)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2230137825_jpg_d14459f501.webp)