Advertisement|Remove ads.
Insmed Stock Soars Pre-market After Lung Disease Drug Achieves Positive Results In Mid-Stage Trial: Retail Gets More Bullish

Shares of Insmed Incorporated (INSM) soared 25% in Tuesday’s pre-market after the company announced positive results from a mid-stage study evaluating its drug for the treatment of pulmonary arterial hypertension.
Pulmonary arterial hypertension (PAH) is a progressive condition in which the small blood vessels in the lungs become narrowed, resulting in high blood pressure in the pulmonary arteries.
Insmed was evaluating the efficacy and safety of Treprostinil Palmitil Inhalation Powder (TPIP) in patients diagnosed with the condition.
The mid-stage study, involving 102 patients, demonstrated that the drug achieved a 35% placebo-adjusted reduction in pulmonary vascular resistance, or the resistance to blood flow through the pulmonary vasculature, from baseline.
It also reduced levels of a protein produced by the heart's ventricles in response to increased stress or volume overload by 60%.
Treatment-emergent adverse events (TEAEs) occurred in 88.4% of patients who received TPIP, compared to 75.8% of patients who received placebo. Serious TEAEs were observed in 7.2% of patients who received TPIP, versus 3.0% of patients who received placebo.
TEAEs leading to treatment discontinuation were experienced by 5.8% of patients taking TPIP, compared to none in the placebo group, the company stated. The firm emphasized that no deaths occurred during the study.
Insmed said that it will immediately engage with the U.S. Food and Drug Administration (FDA) regarding a late-stage study design following the positive results from the mid-stage trial.
The company plans to initiate a late-stage trial in patients with pulmonary hypertension associated with interstitial lung disease (PH-ILD), a group of conditions that cause scarring and thickening of the lung tissue, before the end of 2025, and a late-stage trial in patients with PAH in early 2026.
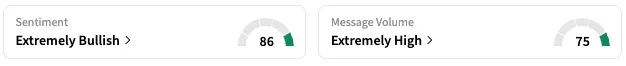
On Stocktwits, retail sentiment around Insmed jumped from ‘bullish’ to ‘extremely bullish’ territory over the past 24 hours while message volume rose from 'high' to 'extremely high' levels.
A Stocktwits user expressed optimism for the company’s product pipeline, including TPIP.
INSM stock is up by about 1% this year and 18% over the past 12 months.
Read Next: Uber To Test Robotaxis In London With AI-startup Wayve: Retail Sees Stock Touching ‘Triple Digits’
For updates and corrections, email newsroom[at]stocktwits[dot]com.









/filters:format(webp)https://news.stocktwits-cdn.com/Getty_Images_1247400381_jpg_765e6ec016.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/vivekkrishnanphotography_58_jpg_0e45f66a62.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Black_Rock_Bitcoin_ETF_IBIT_f66b744bfc.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/Anushka_Basu_make_me_smile_in_the_picture_b92832aa_af59_4141_aacc_4180d2241ba8_1_2_png_1086e0ed8c.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_kraken_2091850a33.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_wall_street_resized1_jpg_7f200ce842.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/Rounak_Author_Image_7607005b05.png)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2215666275_jpg_07d03239b9.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/shivani_photo_jpg_dd6e01afa4.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_bitcoin_2026_2_jpg_a7bbca2bde.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/Prabhjote_DP_67623a9828.jpg)