Advertisement|Remove ads.
Kura Oncology Stock Ignites Retail Frenzy After FDA Clears First Daily Pill For Relapsed Leukemia Patients

- The FDA approved KOMZIFTI, a once-daily oral therapy for relapsed or refractory NPM1-mutated AML.
- Kura and Kyowa Kirin said the treatment will be available immediately under their joint commercialization plan.
- Stocktwits users pointed to milestone-based deal terms and said Kura’s pipeline remains undervalued.
Retail buzz spiked for Kura Oncology on Thursday after the FDA granted full approval to KOMZIFTI, the first once-daily oral treatment for adults with relapsed or refractory acute myeloid leukemia (AML) driven by an NPM1 mutation. The decision came ahead of the agency’s target action date of Nov.30.
FDA Approves New Option For Patients With Limited Alternatives
The approval gives patients with this fast-moving form of AML a simple daily pill that can be taken at home, offering an alternative to intensive chemotherapy regimens that many are unable to tolerate. NPM1 mutations are among the most common genetic drivers of AML, and once the disease returns, effective treatment options become limited.
Approval was supported by the KOMET-001 trial, which tested KOMZIFTI in 112 adults with the disease who had a recurrence or did not respond to prior treatment. Some patients achieved complete remission or partial hematologic recovery, with most responses occurring within the first six months. The FDA also noted that the drug can be used with most supportive medicines and does not carry a warning about heart rhythm risks.
Kura and Kyowa Kirin Begin Commercial Rollout
Kura and its partner Kyowa Kirin said they are ready to make KOMZIFTI available immediately. Under their collaboration, Kura will lead development, regulatory efforts, and commercial strategy in the U.S., while Kyowa Kirin will handle markets outside the U.S. Both companies will coordinate certain promotional activities through a joint commercialization plan. Additionally, a patient-support program will be implemented to improve access and reimbursement.
Stocktwits Traders See Room For Pipeline Re-Rating
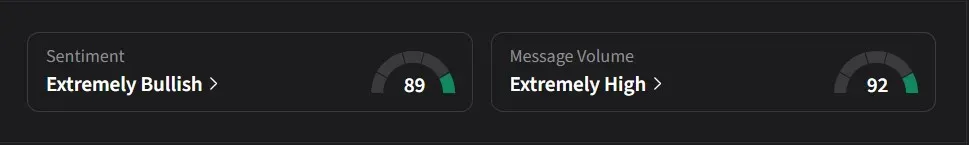
On Stocktwits, retail sentiment for Kura was ‘extremely bullish’ amid a 3,725% surge in 24-hour message volume.
One user said the Kura-Kyowa Kirin agreement involves milestone-based payments rather than a single upfront sum, with both companies set to share profits once sales begin. They said Kura appears well-funded, has already prepared for launch, and could see added momentum as approvals expand outside the U.S.
Another user compared Kura’s valuation with peers, noting that both Kura and Syndax advanced similar therapies through Phase 2 at comparable market caps. They said the market has yet to fully reflect the value of Kura’s broader pipeline.
Kura Oncology’s stock has risen 75% so far in 2025.
For updates and corrections, email newsroom[at]stocktwits[dot]com.













/filters:format(webp)https://news.stocktwits-cdn.com/large_ethereum_blue_original_jpg_b6e7cc57f6.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/Prabhjote_DP_67623a9828.jpg)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Novo_Nordisk_jpg_96dd19f953.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/Rounak_Author_Image_7607005b05.png)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/large_microstrategy_michael_saylor_resized_9fd19e69ec.jpg)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2262651778_jpg_54075aa1d9.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/IMG_9209_1_d9c1acde92.jpeg)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/large_ibm_signage_mwc_resized_28f91e1a63.jpg)
/filters:format(webp)https://news.stocktwits-cdn.com/large_circle_stablecoins_original_jpg_b238d12be8.webp)