Advertisement|Remove ads.
MLTX Stock Extends Rally As FDA Feedback Spurs Bullish Brokerage Action – Retail Speculates On Potential M&A Conversations

- H.C. Wainwright raised MoonLake’s price target to $32 from $26 and maintained a ‘Buy’ rating.
- BTIG said the FDA approval ‘clears a significant overhang’ for MoonLake’s clinical program.
- MoonLake plans to submit its biologics license application in the second half of 2026.
MoonLake Immunotherapeutics (MLTX) remained on investors’ radar after its shares rose 5% in premarket trading on Friday, following favorable brokerage action tied to positive feedback from the U.S. Food and Drug Administration (FDA) on its treatment for hidradenitis suppurativa, a chronic inflammatory skin condition.
MLTX was among the top trending tickers on Stocktwits at the time of writing.
BTIG upgraded MoonLake Immunotherapeutics to ‘Buy’ from ‘Neutral’ and set a $24 price target, citing a recent regulatory update, according to TheFly.
The FDA Feedback
The FDA confirmed that MoonLake can establish the effectiveness of Sonelokimab (SLK) in hidradenitis suppurativa without additional trials, a development BTIG believes clears a significant overhang for the company’s clinical program and could position SLK for FDA approval.
This would be supported by existing data from the VELA-1, VELA-2, and MIRA studies. The company plans to submit its biologics license application in the second half of 2026.
H.C. Wainwright raised MoonLake’s price target to $32 from $26 and maintained a ‘Buy’ rating. The company’s FDA meeting outcome delineates a path to registration, the firm said.
The stock closed 27% higher on Thursday following the FDA feedback.
How Did Stocktwits Users React?
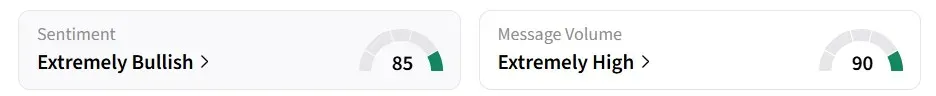
Retail sentiment on Stocktwits remained in the ‘extremely bullish’ zone over the past 24 hours, accompanied by ‘extremely high’ message volumes.
One user sees potential conversations regarding mergers and acquisitions following the FDA feedback.
Another user believes MLTX’s value needs to grow further before a probable acquisition.
MoonLake had reportedly turned down a $3 billion acquisition offer from Merck (MRK) in June last year.
The stock has been under heavy selling pressure over the past year, declining more than 70%.
Read also: GLUE Stock Slips Pre-Market After Pricing $300 Million Public Offer At Discount
For updates and corrections, email newsroom[at]stocktwits[dot]com.













/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2228875477_jpg_4c76a2e8b7.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/vivekkrishnanphotography_58_jpg_0e45f66a62.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2213365850_jpg_470b9c6c06.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/Rounak_Author_Image_7607005b05.png)
/filters:format(webp)https://news.stocktwits-cdn.com/large_michael_saylor_strategy_2013_resized_jpg_e358c15fd4.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/Prabhjote_DP_67623a9828.jpg)
/filters:format(webp)https://news.stocktwits-cdn.com/large_lithium_47e0215e10.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_evolent_jpg_3c3f2aa8e5.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2250655281_jpg_c8c0e9352f.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/shivani_photo_jpg_dd6e01afa4.webp)