Advertisement|Remove ads.
Moderna Retains Retail Support With European COVID-19 Vaccine Tender Win, Stock Hits 2-Week High

Shares of Moderna Inc. climbed nearly 1% on Friday, reaching levels last seen on Jan. 10, as retail sentiment turned increasingly positive following the company’s announcement of a new vaccine tender agreement.
Moderna revealed it has secured a tender to supply its mRNA COVID-19 vaccine across the European Union (EU), Norway, and North Macedonia.
The agreement, which spans up to four years, allows 17 participating countries access to Moderna’s vaccine in various formats, including prefilled syringes.
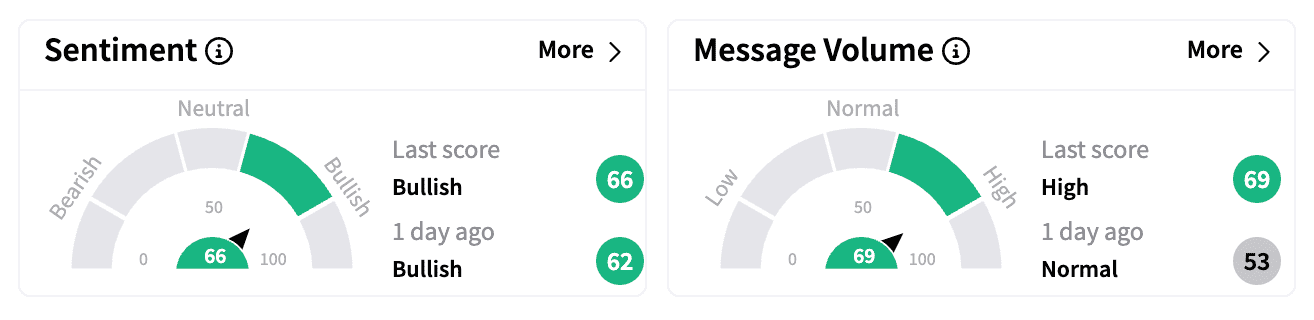
On Stocktwits, sentiment for Moderna remained ‘bullish,’ with increased message volume and discussions around the stock’s potential upside.
Retail traders highlighted the stock’s two-week high and predicted the next resistance level.
The tender win comes amid a mixed period for Moderna. The company recently lowered its 2025 sales guidance by $1 billion due to a weaker outlook for its COVID-19 vaccine business, impacting retail investor sentiment.
However, interest in Moderna’s H5N1 bird flu vaccine candidate has reignited, fueled by growing bird flu cases in the U.S. and a $590 million award from the U.S. Health and Human Services Department to accelerate vaccine development.
Additionally, Moderna’s Spikevax JN.1 vaccine, updated to target a SARS-CoV-2 variant, received marketing authorization from the European Commission in September 2024 for individuals six months and older.
Moderna’s stock has lost over 57% in the past year but is poised for its best weekly gain in nearly three years.
For updates and corrections, email newsroom[at]stocktwits[dot]com.









/filters:format(webp)https://news.stocktwits-cdn.com/large_lucid_gravity_jpg_173d7fb4ec.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/unnamed_jpg_9dff551b50.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/Getty_Images_2250240969_jpg_dd9be8c5ea.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/shivani_photo_jpg_dd6e01afa4.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Trump_jpg_4a30f2c834.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/Rounak_Author_Image_7607005b05.png)
/filters:format(webp)https://news.stocktwits-cdn.com/large_donald_trump_tariffs_chart_jpg_9309f5e523.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2242061511_jpg_742d610600.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/vivekkrishnanphotography_58_jpg_0e45f66a62.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2164981884_1_jpg_100f5d0da3.webp)