Advertisement|Remove ads.
Novo Nordisk Warns Against Counterfeit Ozempic In US: Retail’s Unmoved

Drug maker Novo Nordisk (NVO), on Monday, warned consumers about counterfeit versions of its diabetes drug Ozempic in the U.S. after the Food and Drug Administration (FDA) seized counterfeit drugs last week.
The company said it has become aware of several hundred units of Ozempic (semaglutide) injection 1 mg distributed outside its authorized supply chain in the US.
The company notified the FDA, which seized the counterfeit products on April 9.
The company said that the seized counterfeit products feature lot number PAR0362 and illegitimate serial numbers beginning with the first eight digits, 51746517.
Novo Nordisk said that it is assisting with the FDA’s ongoing investigation and that the chemical testing of the counterfeit product is underway.
The company said neither Novo Nordisk nor the FDA can confirm the counterfeit product's contents or quality, adding that it may pose a safety risk for patients.
It further urged retail pharmacies to purchase Ozempic through authorized distributors of the company and for patients to double-check their Ozempic box upon purchase. The company also urged consumers to report suspicious Ozempic products to the FDA.
NVO shares traded 1% higher on Monday afternoon after rival drug maker Pfizer announced earlier in the day that it is ending the development of its experimental weight loss drug after one patient in its trial group reported a potential drug-induced liver injury, potentially reducing competition for Novo’s Wegovy weight loss drug.
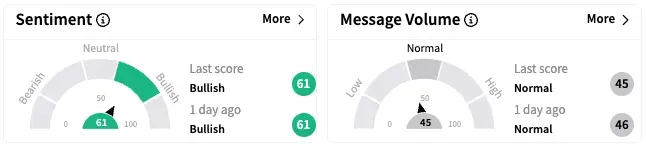
On Stocktwits, retail sentiment around Novo Nordisk stayed unchanged within the ‘bullish’ territory over the past 24 hours while message volume remained at ‘normal’ levels.
NVO stock is down by about 25% year-to-date and about 47% over the past 12 months.
Also See: Apple Tops Global Smartphone Sales In Q1 Led By iPhone 16e, Says Counterpoint
For updates and corrections, email newsroom[at]stocktwits[dot]com












/filters:format(webp)https://news.stocktwits-cdn.com/large_jamie_dimon_jpmorgan_jpg_cbdd07fa63.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/Prabhjote_DP_67623a9828.jpg)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/large_Git_Lab_resized_49b70b74d0.jpg)
/filters:format(webp)https://news.stocktwits-cdn.com/IMG_8805_JPG_6768aaedc3.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_So_Fi_new_6d7889a863.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/IMG_4530_jpeg_a09abb56e6.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_bitcoin_2026_4_jpg_bb96bc484b.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2249860610_jpg_2888fdef75.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_novo_nordisk_ozempic_wegovy_jpg_786cdf3b34.webp)