Advertisement|Remove ads.
Rapport Therapeutics Stock Rockets 204% Pre-Market To Head Toward All-Time Highs – Here’s What Happened

Rapport Therapeutics, Inc. (RAPP) on Monday said that a mid-stage clinical trial of RAP-219 in patients with drug-resistant focal onset seizures demonstrated a statistically significant reduction in long episodes over the 8-week treatment period.
RAP-219 also demonstrated a statistically significant and clinically meaningful reduction in seizures accompanied by physical signs compared with baseline in the study, the company said, while adding that it plans to advance the investigational therapy into two late-stage trials in the third quarter of 2026.
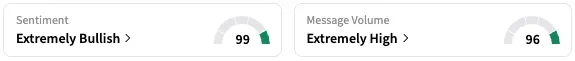
On Stocktwits, retail sentiment around RAPP stock stayed within ‘extremely bullish’ territory over the past 24 hours, while message volume stayed at ‘extremely high’ levels. Shares of the company traded 204% higher in the pre-market session at the time of writing.
A Stocktwits user sounded optimistic, saying the update is ‘great news.’
Another user, however, expressed doubts about whether the data warrants the massive rally.
H.C. Wainwright subsequently raised its price target on Rapport to $34 from $31 while keeping a ‘Buy’ rating on the shares. The firm states that RAP-219 surpassed its "most bullish expectations" and added that the data provides "unimpeachable evidence" of the drug's effect, offering "strong evidence that it has the potential to become a mainstay" in the treatment of focal onset seizures. The firm increased its probability of success for oral RAP-219 to 66% and its peak revenue estimate to $766 million, up from $613 million.
Focal onset seizures are seizures that begin in one area of one brain hemisphere, causing symptoms that typically affect one side of the body or are limited to a specific sensation, emotion, or movement. In the trial, Rapport enrolled 30 patients, 18 men and 12 women, with focal onset seizures who had an implanted RNS System, a brain simulation device for drug-resistant focal epilepsy. The patients received 0.75 mg of RAP-219 oral tablets daily for 5 days, followed by 1.25 mg of RAP-219 oral tablets daily for the remainder of the 8-week treatment period.
During the treatment period, over 85% patients achieved more than or equal to 30% reduction in long episodes from baseline, the company said, while 72% achieved more than or equal to 50% reduction. Twenty-four patients also achieved seizure freedom, the company said. Reduction in long episodes (LEs) is an objective electrographic biomarker for clinical seizure reduction, it said.
RAP-219 was generally well-tolerated in the trial, with the majority of treatment-emergent adverse events being mild and a low discontinuation rate, the company added.
Rapport plans to hold an end-of-mid-stage trial meeting with the U.S. Food and Drug Administration (FDA) in the fourth quarter of 2025. The company also expects to present additional efficacy analyses and 8-week follow-up results in 2026 and to initiate a trial to evaluate the long-term safety of the therapy in patients by the end of 2025. RAP-219 is also being evaluated in bipolar mania and diabetic peripheral neuropathic pain.
RAPP stock is down 19% this year and approximately 27% over the past 12 months.
Read also: PNC To Buy Colorado’s FirstBank for $4.1B In Push To Bolster National Reach: Report
For updates and corrections, email newsroom[at]stocktwits[dot]com.











/filters:format(webp)https://news.stocktwits-cdn.com/large_Robotaxi_Tesla_jpg_209f017098.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/unnamed_jpg_9dff551b50.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/large_AT_and_T_store_resized_542005da9b.jpg)
/filters:format(webp)https://news.stocktwits-cdn.com/Aashika_Suresh_Profile_Picture_jpg_2acd6f446c.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_michael_barr_OG_jpg_6005cfe225.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Stocks_jpg_a3427ddfd2.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/jaiveer_jpg_280ad67f36.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_1900667440_jpg_c3b8e52a81.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/Anushka_Basu_make_me_smile_in_the_picture_b92832aa_af59_4141_aacc_4180d2241ba8_1_2_png_1086e0ed8c.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_1207431426_jpg_b8d7c6d852.webp)