Advertisement|Remove ads.
REPL Stock Nearly Doubled In Value Today: What's Going On?

Shares of Replimune Group Inc. (REPL) were in the retail spotlight on Wednesday after the stock nearly doubled in value.
BMO Capital expects the stock to witness a meaningful rally following the exit of Vinay Prasad from the U.S. Food and Drug Administration, according to TheFly. Prasad stepped down as Director of the Center for Biologics Evaluation and Research (CBER) on Tuesday, nearly three months into the job.
Meanwhile, shares of Capricor Therapeutics (CAPR) and Sarepta Therapeutics (SRPT) clocked moderate gains.
On Stocktwits, retail sentiment around Replimune jumped from ‘bullish’ to ‘extremely bullish’ over the past 24 hours, while message volume stayed at ‘extremely high’ levels.
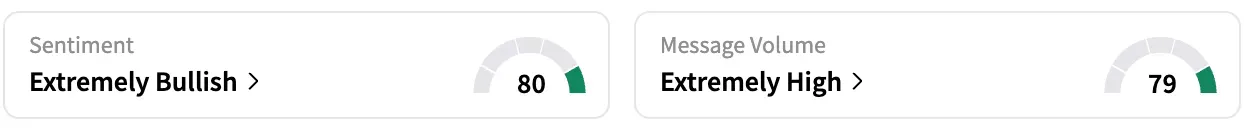
As per Stocktwits data, retail chatter around Replimune surged over 1500% over the past 24 hours.
Prasad was appointed in May to replace Peter Marks, who was removed from CBER after conflicting with Health and Human Services Secretary Robert F. Kennedy Jr. over vaccine policy.
BMO Capital analyst Evan Seigerman views the exit of Vinay Prasad as a positive for the biopharma sector. A new director will "likely be more permissive of innovation than what we have seen to date," the analyst told investors in a research note.
BMO, however, remains uncertain on whether a new CBER director will reconsider the denial of approval to the company’s lead product candidate, RP1.
Earlier this month, Replimune received a letter from the FDA indicating that it is unable to approve the company’s application for RP1 in combination with Nivolumab for the treatment of advanced melanoma.
The agency deemed the trials cited by the company as not adequate to substantiate the effectiveness of the drug. The FDA also said the trial cannot be adequately interpreted due to the heterogeneity of the patient population.
The company then said it will request a meeting with the FDA as it plans to interact with the agency to find a path for approval. CEO Sushil Patel stated that the company is surprised and disappointed by the decision.
Leerink, meanwhile, views the exit of Prasad as a positive for rare disease and genetic medicines, negative for vaccines, and unclear for oncology, as per TheFly. While Prasad restricted the use of COVID-19 vaccines during his tenure, Leerink expects the new chief to be more in alignment with Robert F. Kennedy Jr. and his anti-vaccine stance.
Shares of Capricor Therapeutics also traded 25% higher on Wednesday. The FDA had earlier refused to approve its lead cell therapy candidate for the treatment of cardiomyopathy associated with Duchenne muscular dystrophy.
For updates and corrections, email newsroom[at]stocktwits[dot]com.










/filters:format(webp)https://news.stocktwits-cdn.com/large_Stock_chart_representative_image_resized_jpg_dacf5b1590.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/vivekkrishnanphotography_58_jpg_0e45f66a62.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Trump2_jpg_ad63f384b5.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/Rounak_Author_Image_7607005b05.png)
/filters:format(webp)https://news.stocktwits-cdn.com/Getty_Images_1247400381_jpg_765e6ec016.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/shivani_photo_jpg_dd6e01afa4.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_bitcoin_atm_original_jpg_afc73e9be7.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/Prabhjote_DP_67623a9828.jpg)
/filters:format(webp)https://news.stocktwits-cdn.com/large_GE_Aerospace_resized_1_jpg_03f4fd7e4e.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2260447662_jpg_cd246b74f6.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/IMG_9209_1_d9c1acde92.jpeg)