Advertisement|Remove ads.
Sarepta Stock Slumps After EU Regulator Recommends Refusing Approval To Its Contentious Gene Therapy: Retail Expects Stock Bounceback

The European Medicines Agency (EMA) on Thursday recommended the refusal of marketing authorisation for Sarepta Therapeutics’ (SRPT) gene therapy Elevidys, a medicine intended for the treatment of Duchenne muscular dystrophy (DMD), as it faces heightened scrutiny in the U.S.
Roche (RHHBY) had applied for approval of the drug in the EU. While Sarepta is responsible for the regulatory approval and commercialization of Elevidys in the U.S., Roche is responsible for regulatory approvals worldwide.
Roche announced on Friday that it plans to continue working with the EMA to explore a potential path forward.
"We are disappointed by the CHMP’s (Committee for Medicinal Products for Human Use) negative opinion, given the urgent need for disease-modifying therapies for children in the EU living with Duchenne," said Levi Garraway, Chief Medical Officer at Roche.
The company also said that it believes the benefit-risk profile for Elevidys remains positive in the ambulatory Duchenne population.
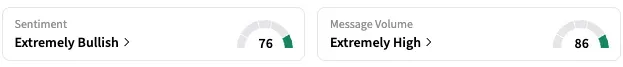
On Stocktwits, retail sentiment around Sarepta is trending in the ‘extremely bullish’ territory, coupled with ‘extremely high’ message volume. According to data from the platform, retail chatter about the stock has increased by 529% over the past 24 hours.
Elevidys is already approved in the U.S. However, earlier this week, Sarepta announced a voluntary halt to U.S. shipments of Elevidys following a request from the FDA after a patient receiving a different experimental treatment from the company died. Sarepta is also updating safety labeling on Elevidys following two earlier deaths from liver failure in non-ambulatory patients with advanced-stage DMD.
The shipment pause, effective Tuesday evening, is intended to give Sarepta time to address FDA information requests and finalize the updated safety label.
“Following the initial FDA approval of ELEVIDYS on June 22, 2023, the therapy has subsequently received regulatory approval in several other countries. In the U.S., we are actively working with the FDA to address recent safety questions. We remain committed to working with regulators to address outstanding questions on safety so that people living with Duchenne have access to this important therapy,” said Louise Rodino-Klapac, president of research & development and technical operations, Sarepta.
Duchenne Muscular Dystrophy is a genetic disorder characterized by progressive weakness and chronic inflammation of the skeletal, heart, and respiratory muscles. The disease affects approximately 15,000 individuals in the United States and primarily impacts boys.
A Stocktwits user expressed optimism that Roche will handle the situation.
Another said that they expect the stock to bounce back soon.
SRPT stock is down by over 90% this year and by about 92% over the past 12 months.
Read Next: Centene Reports Unexpected Q2 Loss: But Retail Expects Stock To Close Higher
For updates and corrections, email newsroom[at]stocktwits[dot]com.













/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2227231004_jpg_0de480c6f4.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/Aashika_Suresh_Profile_Picture_jpg_2acd6f446c.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2254924041_jpg_892ccf911d.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/jaiveer_jpg_280ad67f36.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/Getty_Images_1234736300_jpg_881ee00045.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2218899763_jpg_5d8b51f97d.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/Prabhjote_DP_67623a9828.jpg)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Trump_golden_dome_resized_jpg_38d699de1f.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2222922178_jpg_42dd8f319f.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/unnamed_jpg_9dff551b50.webp)