Advertisement|Remove ads.
Travere Stock Nosedives As FDA Schedules Advisory Meeting For Kidney Drug's Supplemental Use: Retail Shrugs Off Worries
Travere Therapeutics shares tumbled over 15% in extended trading Thursday after the company said the U.S. Food and Drug Administration will convene an advisory committee meeting to review a supplemental use of its kidney drug, Filspari.
The FDA accepted Travere's supplemental New Drug Application (sNDA) seeking traditional approval of Filspari for the treatment of focal segmental glomerulosclerosis (FSGS), a rare and serious kidney disorder with no FDA-approved therapies.
Under the Prescription Drug User Fee Act, the agency has now set a target action date of Jan. 13, 2026.
"We are one step closer to potentially delivering the first approved treatment for people living with FSGS - a leading cause of kidney failure and devastating condition that urgently needs new treatment options," said CEO Eric Dube.
While the advisory committee (AdComm) meeting introduces an added review step, it is a standard part of the FDA's drug approval process and not necessarily a red flag. However, negative recommendations from such meetings can lead to delays or rejections.
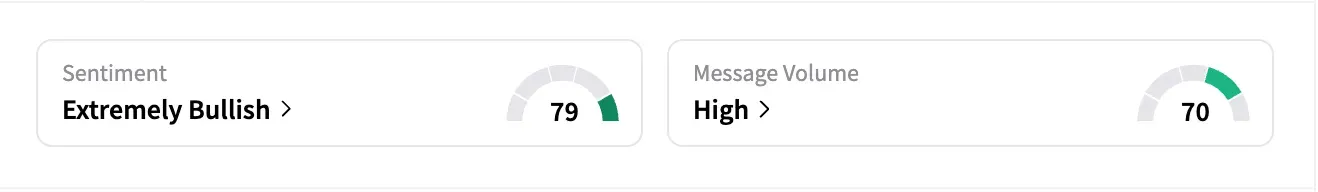
Despite the market reaction, sentiment on Stocktwits ended 'extremely bullish' on Thursday, with message volume jumping from 'normal' to 'high.' Some retail traders saw the selloff as overdone.
"Oversold now. It's an ADCOM, and by no means a bad thing when a meeting is required. It boils down to a safe and highly needed prescription drug, that just needs to thread the needle of the FDA," one user wrote.
"From late fall to just January 2026, is not that big of a deal to wait to get the "T's" crossed and the "I's" dotted. A few months added from the original timeframe," another said, brushing off the timeline shift.
However, investors often cheer when the FDA skips an AdComm — as seen in March, when Tonix Pharma stock surged on news its fibromyalgia drug would not require such a review.
Filspari is already approved to slow kidney function decline in IgA nephropathy, another rare kidney condition. FSGS affects more than 40,000 patients in the U.S., and Travere is seeking to expand Filspari's label for this population.
Travere shares are up over 19% year to date, but short interest has climbed, rising from 8.5% at the start of 2025 to 11.9% as of last week, according to Koyfin.
For updates and corrections, email newsroom[at]stocktwits[dot]com.














/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2262606802_1_jpg_86ff244e32.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/IMG_4530_jpeg_a09abb56e6.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/Getty_Images_2250929484_jpg_8206df84ab.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/IMG_8805_JPG_6768aaedc3.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2215390052_jpg_84ddd1faac.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/IMG_9209_1_d9c1acde92.jpeg)
/filters:format(webp)https://news.stocktwits-cdn.com/Getty_Images_2177851484_jpg_b969f68c05.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/Getty_Images_2158238458_jpg_48ab7af27c.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Rocketlab_resized_jpg_92c1a02a7f.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/unnamed_jpg_9dff551b50.webp)