Advertisement|Remove ads.
Travere Therapeutics Sparks Retail Frenzy As Stock Hits Over 2-Year High On FDA Approval Plan For Kidney-Disease Drug
Shares of Travere Therapeutics Inc. surged over 13% Tuesday afternoon, reaching their highest level since October 2022 and driving a spike in retail discussions on Stocktwits.
The rally followed Travere’s announcement that it plans to seek full FDA approval for Filspari in treating focal segmental glomerulosclerosis (FSGS), a rare kidney disorder.
The company expects to submit a supplemental New Drug Application (sNDA) based on existing Phase 3 and Phase 2 data by the end of the first quarter.
According to The Fly, Wells Fargo sees the accelerated timeline as a strong positive. It estimates a 60%- 70% probability of success, which it models as a fair value range of $27-$30 per share.
At full approval, the firm values the stock at $40 per share, but currently maintains an ‘Overweight’ rating with a $27 price target.
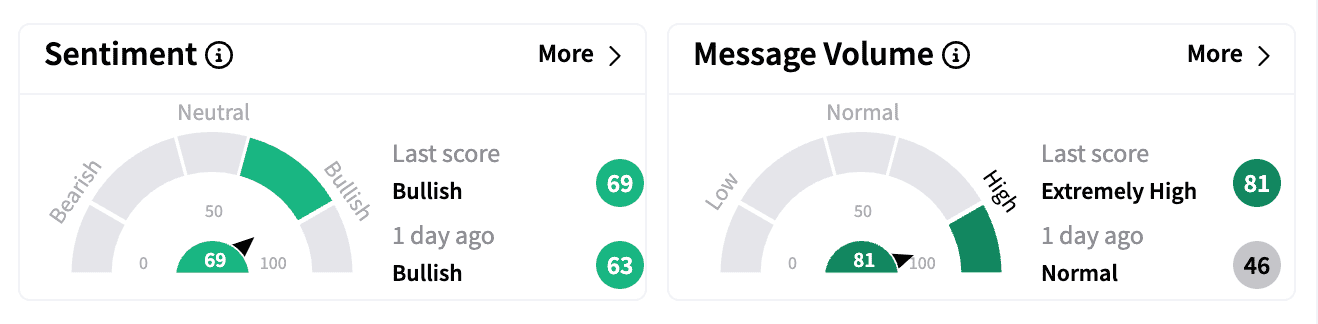
Stocktwits sentiment for Travere turned ‘bullish,’ with message volume (81/100) hitting a three-month high.
Retail traders cheered the news, with some analyzing technical patterns signaling further gains.
FSGS is a rare kidney disorder affecting over 40,000 patients in the U.S., with no current FDA-approved treatments.
Filspari has already been approved to slow kidney function decline in adults with IgA nephropathy.
Shares of Travere have nearly tripled over the past 12 months.
For updates and corrections, email newsroom[at]stocktwits[dot]com.









/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/large_stock_down_resized_901c19c371.jpg)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/unnamed_jpg_9dff551b50.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/Getty_Images_2218096416_jpg_9d469a2ec6.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/jaiveer_jpg_280ad67f36.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/large_sealsq_stock_market_representative_resized_b05435011f.jpg)
/filters:format(webp)https://news.stocktwits-cdn.com/Getty_Images_2229019640_jpg_c6006d7238.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/large_adam_smigielski_K5m_Pt_O_Nmp_HM_unsplash_f365ee93f4.jpg)
/filters:format(webp)https://news.stocktwits-cdn.com/large_trump_jpg_fc59d30bbe.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/Aashika_Suresh_Profile_Picture_jpg_2acd6f446c.webp)