Advertisement|Remove ads.
Verastem Stock Ignites Retail Buzz After Early Cancer Drug Data Show Tumor Reduction, Mild Side Effects

- Verastem reported early trial data showing its experimental oral therapy targeting KRAS-driven cancers was well-tolerated, with no severe gastrointestinal side effects.
- Four of five evaluable patients showed tumor reduction, prompting optimism around the drug’s potential for pancreatic and colorectal cancer treatment.
- Retail traders on Stocktwits responded enthusiastically, calling the update an example of improved, patient-focused design and praising the drug’s tumor-reducing effects.
Verastem Oncology shares drew heavy retail chatter on Thursday after the company reported encouraging early results from its Phase 1/2a trial of VS-7375, an investigational oral KRAS G12D inhibitor targeting hard-to-treat solid tumors such as pancreatic and colorectal cancers.
Early Safety Data And Tumor Response
The company said the first two monotherapy dose levels of 400 mg and 600 mg once daily were cleared with no dose-limiting toxicities, and no nausea, vomiting, or diarrhea above Grade 1 was observed. Of the five patients evaluable for efficacy, four experienced tumor reduction and remain on treatment.
“Preliminary safety and tolerability data suggest VS-7375 can be administered at efficacious doses while effectively managing gastrointestinal side effects,” said CEO Dan Paterson, adding that the company is seeing “anti-tumor activity among pre-treated patients with advanced pancreatic cancer and other solid tumors.”
Combination Study Now Enrolling
With monotherapy dose escalation continuing to 900 mg, Verastem said it has begun enrolling patients in a combination cohort testing VS-7375 with cetuximab in KRAS G12D-mutant solid tumors, including colorectal cancer.
Verastem’s Chief Medical Officer John Hayslip said the company is encouraged by the drug’s tolerability and early signs of anti-tumor activity, adding that the ongoing combination trial with cetuximab addresses “a significant unmet need for treatments specifically targeting KRAS G12D-mutant cancers.”
The company plans to report an interim safety and efficacy update in the first half of 2026 and expand the trial globally following dose optimization.
Background On The Candidate
VS-7375 is the lead program from Verastem’s collaboration with GenFleet Therapeutics, which is running a parallel study in China under the name GFH375. GenFleet previously selected 600 mg once daily as the recommended Phase 2 dose, citing similar safety and response patterns.
The KRAS G12D mutation accounts for 26% of all KRAS mutations and is most prevalent in pancreatic, colorectal, endometrial, and lung cancers, which have few or no targeted treatment options.
Stocktwits Bulls Cheer Early Trial Results
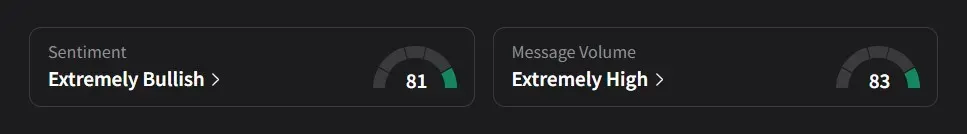
On Stocktwits, retail sentiment for Verastem was ‘extremely bullish’ amid a 123% surge in 24-hour message volume.
One user said they were encouraged by Verastem’s latest update, calling it an example of how small refinements in trial design and dosing can meaningfully improve patient experience and tolerability.
Another user expressed optimism about Verastem, noting that the drug appeared to reduce tumor size while causing limited side effects.
Verastem’s stock has risen 51% so far in 2025.
For updates and corrections, email newsroom[at]stocktwits[dot]com.














/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_540185275_jpg_21d1350875.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/shivani_photo_jpg_dd6e01afa4.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_justin_sun_resized_jpg_80141eb4e8.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/Anushka_Basu_make_me_smile_in_the_picture_b92832aa_af59_4141_aacc_4180d2241ba8_1_2_png_1086e0ed8c.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2242061511_jpg_742d610600.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/vivekkrishnanphotography_58_jpg_0e45f66a62.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/large_eli_lilly_hq_resized_af4cc05fd5.jpg)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/Rounak_Author_Image_7607005b05.png)
/filters:format(webp)https://news.stocktwits-cdn.com/Getty_Images_2204154647_jpg_b295df5f6b.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_MSTR_caaa0be909.webp)