Advertisement|Remove ads.
Wave Life Sciences Stock Rockets 120% After Obesity Drug Shows Deep Fat Reduction, Muscle Gain — Traders See A ‘Blockbuster' Candidate

- Single-dose results showed clear shifts in visceral fat, total fat, and lean mass at three months.
- Activin E levels dropped sharply and remained suppressed through Day 85.
- WVE-007 was generally well tolerated with no severe or serious adverse events reported.
Shares of Wave Life Sciences (WVE) jumped over 120% on Monday after the company released positive interim results from its Phase 1 INLIGHT trial evaluating WVE-007, an investigational INHBE-targeting GalNAc-siRNA for obesity.
Results At Three Months
Wave said that a single 240 mg dose of WVE-007 produced clear changes in body composition by the three-month check. On average, participants saw a 9.4% drop in visceral fat, a 4.5% reduction in total body fat, or 3.5 lbs, and a 3.2% increase in lean mass, or 4 lbs. In the placebo group, the corresponding changes were a 0.2% decrease in visceral fat, a 0.5% decrease in total body fat, and a 2.3% increase in lean mass.
The company highlighted consistent and durable suppression of serum Activin E, with maximum reductions of 78% observed 43 days after dosing and mean reductions above 75% maintained through Day 85. Wave said the durability of these reductions supports a potential dosing schedule of once or twice per year.
Wave described WVE-007 as generally safe and well tolerated up to 600 mg. There were no discontinuations or severe or serious treatment-emergent adverse events. All treatment-related events were mild. There were no clinically meaningful changes from baseline in lipid profiles or liver function tests.
Genetic Rationale
Wave noted that individuals with a natural loss-of-function variant in one copy of the INHBE gene tend to have healthier body composition, lower visceral fat, and reduced risk of type 2 diabetes and cardiovascular disease. The company said this human genetics foundation supports the therapeutic potential of WVE-007.
Phase 2 Planning
The company said that planning was ongoing for Phase 2 studies evaluating WVE-007 as a monotherapy, as an add-on to incretin therapies and as a maintenance therapy after stopping incretin treatment. More clinical updates are expected in the first quarter of 2026, including six-month data from the 240 mg cohort and three-month data from the 400 mg cohort.
Wave also said that in the second quarter of 2026, it expects to report six-month follow-up data from the 400 mg single-dose cohort and three-month follow-up data from the 600 mg single-dose cohort.
Analyst Takes
Leerink called the update “an exciting first look,” saying the data build on Wave’s prior Activin E reductions and show the preclinical findings are “translating very nicely,” while maintaining an 'Outperform' rating.
Jefferies said the lowest-dose data met its base case and offered “incremental confidence” for later readouts, reiterating its 'Buy' rating and $26 target. Truist said the results “fundamentally" improve the obesity landscape outlook and validate Wave’s siRNA platform, keeping a 'Buy' rating and $36 target.
Stocktwits User See A 'Blockbuster' Moment
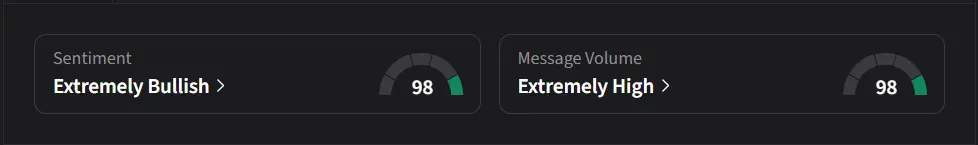
On Stocktwits, retail sentiment for Wave was ‘extremely bullish’ amid ‘extremely high’ message volume.
One user said, “based on the data this can become a blockbuster investment.”
Another user claimed, “these are exactly the kind of stocks that bust through 1000% in a matter of days. An easy hold for me and I’m loading on those dips!!”
Wave’s stock has risen 33% so far in 2025.
For updates and corrections, email newsroom[at]stocktwits[dot]com.











/filters:format(webp)https://news.stocktwits-cdn.com/large_tesla_cybertruck_jpg_7f6ed70b80.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/unnamed_jpg_9dff551b50.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Nebius_jpg_291bb409c7.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/Aashika_Suresh_Profile_Picture_jpg_2acd6f446c.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2202349941_jpg_3f45878d03.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/large_marathon_holdings_resized_40790d98cc.jpg)
/filters:format(webp)https://news.stocktwits-cdn.com/jaiveer_jpg_280ad67f36.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_trump_jpg_fc59d30bbe.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_nio_es8_jpg_6097d170b7.webp)