Advertisement|Remove ads.
Why Are Retail Traders Going Gaga Over BioCryst Pharma Stock Today?

- BioCryst said the approval follows positive interim data from a trial that showed the drug is safe and well-tolerated.
- The company is also seeking approval for the drug’s use in the targeted age group in Europe, Japan, Canada, and other countries.
- Orladeyo in capsule form is already FDA-approved for treating the disorder in those over 12 years of age.
BioCryst Pharmaceuticals, Inc. (BCRX) has received approval from the U.S. Food and Drug Administration (FDA) for Orladeyo, an oral pellet formulation for the treatment of a rare genetic disorder in children.
The approval comes after positive interim data from the APeX-P trial, which showed it is safe and well-tolerated and effectively reduces monthly HAE attacks in young children, the company said in a statement.
BioCryst has also filed an application for the drug’s use with the European Medicines Agency and the Japan Pharmaceutical and Medical Devices Agency. The company said it is exploring additional regulatory filings in other global territories, including Canada.
Shares of BCRX jumped over 4% on Friday morning at the time of writing.
Breakthrough Treatment For Young Children
Hereditary angioedema (HAE) is a rare genetic disorder that can cause sudden and severe swelling in the skin, face, limbs, gut, and air pathways with potential life-threatening consequences.
Orladeyo will become the first approved oral drug for the treatment of hereditary angioedema (HAE) in children aged 2 to 12, the company said. All other treatments for HAE in the market for the targeted demographic are currently administered intravenously or through injections.
A capsule formulation of Orladeyo for treating HAE in children over 12 years and older adults received FDA approval in December 2020.
“As we mark five years since ORLADEYO first transformed care for those with HAE ages 12 and older, this approval extends the benefits of oral prophylactic therapy to a vulnerable and important part of the HAE community, children ages 2 to less than 12. We couldn’t be more excited to bring this treatment option to these kids and their caregivers,” said Jon Stonehouse, chief executive officer of BioCryst.
How Did Stocktwits Users React?
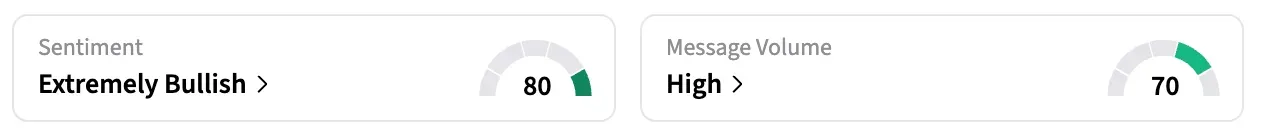
On Stocktwits, BCRX was among the top five trending stocks on Friday morning. The retail sentiment around BCRX jumped from ‘bullish’ to ‘extremely bullish’ territory over the past 24 hours, while message volume remained ‘high’ at the time of writing.
One Stocktwits user commended the FDA news.
Shares of BCRX are down over 3% this year.
For updates and corrections, email newsroom[at]stocktwits[dot]com.











/filters:format(webp)https://news.stocktwits-cdn.com/Getty_Images_1247400381_jpg_765e6ec016.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/vivekkrishnanphotography_58_jpg_0e45f66a62.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Black_Rock_Bitcoin_ETF_IBIT_f66b744bfc.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/Anushka_Basu_make_me_smile_in_the_picture_b92832aa_af59_4141_aacc_4180d2241ba8_1_2_png_1086e0ed8c.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_kraken_2091850a33.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_wall_street_resized1_jpg_7f200ce842.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/Rounak_Author_Image_7607005b05.png)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2215666275_jpg_07d03239b9.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/shivani_photo_jpg_dd6e01afa4.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_bitcoin_2026_2_jpg_a7bbca2bde.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/Prabhjote_DP_67623a9828.jpg)