Advertisement|Remove ads.
Why Did ATOS Stock Jump 10% In Pre-Market Today?

- ODD is granted to a drug or biological product intended to prevent, diagnose, or treat a rare disease or condition.
- The designation provides Atossa with incentives such as tax credits for qualified clinical trial expenses, exemptions from certain FDA user fees, and potential for seven years of market exclusivity.
- (Z)-Endoxifen is a targeted therapy developed to disrupt estrogen signaling by inhibiting and degrading estrogen receptors.
Shares of Atossa Therapeutics (ATOS) rose 10% in premarket trading on Friday, after the U.S. Food and Drug Administration (FDA) granted Orphan Drug Designation (ODD) to its investigational therapy (Z)-Endoxifen for Duchenne Muscular Dystrophy.
Importance Of ODD Approval
The FDA can grant ODD to a drug or biological product intended to prevent, diagnose, or treat a rare disease or condition.
The designation provides sponsors (Atossa) with several incentives, including tax credits for qualified clinical trial expenses, exemptions from certain FDA user fees, and the potential for seven years of market exclusivity following approval. ODD is a separate process from seeking approval or licensing.
Last week, the FDA issued a “Study May Proceed” letter for the company’s metastatic breast cancer trial under an IND for (Z)-endoxifen, a decision the company said supports expanding the drug’s use in ER+/HER2- metastatic breast cancer.
What Is This Investigational Drug?
(Z)-Endoxifen is a selective estrogen receptor modulator designed to inhibit and degrade estrogen receptors, including hormone-resistant cancers. The company is also exploring the drug’s broader therapeutic potential across additional disease pathways. The pharmaceutical company is developing an oral formulation designed to pass through the stomach unchanged, so it doesn’t become inactive.
How Did Stocktwits Users React?
Retail sentiment for ATOS on Stocktwits remained in the ‘bearish’ territory over the past 24 hours, despite the sharp premarket climb.
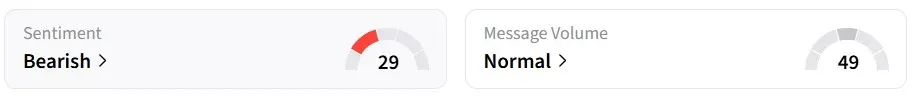
One user believes that the news could push the stock price above $1. It is currently at $0.6.
Atossa will hold a shareholder meeting on January 20 to propose, among other items, a reverse split of its common stock at a ratio of 5:1 to 20:1.
For updates and corrections, email newsroom[at]stocktwits[dot]com












/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2229918735_jpg_e905cbd5e3.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/Aashika_Suresh_Profile_Picture_jpg_2acd6f446c.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_1445160636_jpg_9759816169.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/unnamed_jpg_9dff551b50.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2250240977_jpg_5b777d96ef.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/jaiveer_jpg_280ad67f36.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2206295220_jpg_1057588802.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/Rounak_Author_Image_7607005b05.png)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Trending_stock_resized_may_jpg_bc23339ae7.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/vivekkrishnanphotography_58_jpg_0e45f66a62.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2247614893_jpg_e1dcf2d2f6.webp)