Advertisement|Remove ads.
Why Did DRUG Stock Rocket 51% Today?

- The trial had 24 patients, exceeding the original target of 20, and included a 4-week baseline.
- In the Absence cohort, patients experienced a median 73.1% reduction in seizures.
- The DEE cohort saw a median 63.3% decrease in major motor seizures.
Shares of Bright Minds Biosciences Inc. (DRUG) rose over 51% on Tuesday morning, after the company announced promising topline results from its Phase 2 BREAKTHROUGH trial evaluating BMB-101.
BMB-101 is a selective 5-HT2C biased agonist, in adults with drug-resistant Absence Seizures and Developmental and Encephalopathic Epilepsies (DEE). The study met its primary goals, showing strong reductions in seizure frequency while maintaining a favorable safety profile.
Trial And Participants
The trial had 24 patients, exceeding the original target of 20, and included a 4-week baseline, 4-week titration, and maintenance period consisting of 2 weeks for the Absence cohort and 4 weeks for the DEE cohort.
Participants were heavily pretreated, with a median of 3 anti-seizure medications in the Absence cohort and 5 in the DEE cohort, having failed multiple prior therapies.
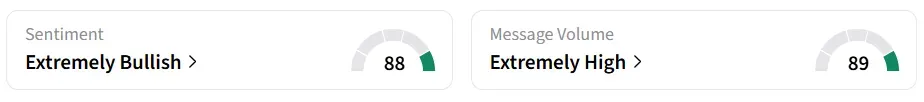
On Stocktwits, retail sentiment around the stock jumped to ‘extremely bullish’ from ‘bearish’ territory. Message volume improved to ‘extremely high’ from ‘low’ levels in 24 hours.
Seizure Reduction
In the Absence cohort, patients experienced a median 73.1% reduction in seizures lasting more than or equal to three seconds and a 74.4% decrease in total seizure time over 24 hours.
The DEE cohort, including patients with Lennox-Gastaut Syndrome (LGS), Dravet syndrome, and Rett syndrome, saw a median 63.3% decrease in major motor seizures. LGS patients achieved a 60.3% reduction, while other DEE participants experienced a 76.1% reduction.
Beyond seizure control, BMB-101 increased REM sleep by 90%, from 56.2 to 106.7 minutes, without altering total sleep duration.
DRUG stock has gained over 93% in the last 12 months.
Also See: Why Did ARWR Stock Surge Over 15% Pre-Market Today?
For updates and corrections, email newsroom[at]stocktwits[dot]com.











/filters:format(webp)https://news.stocktwits-cdn.com/large_uniqure_jpg_33b6552285.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/vivekkrishnanphotography_58_jpg_0e45f66a62.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Sharp_Link_Gaming_jpg_60ce5684e3.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/Anushka_Basu_make_me_smile_in_the_picture_b92832aa_af59_4141_aacc_4180d2241ba8_1_2_png_1086e0ed8c.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_new_york_stock_exchange_jpg_e1f85c0d8c.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/Rounak_Author_Image_7607005b05.png)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2243050664_jpg_37b52748e2.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_hims_stock_logo_resized_jpg_5554a2a2c1.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Oil_drill_06147e8349.webp)