Advertisement|Remove ads.
Why Did Praxis Precision Stock Surge 12% Pre-Market Today?

- The early clinical evidence suggested ulixacaltamide could provide meaningful improvement over current therapies for ET patients.
- The BTD recognizes ulixacaltamide as a potential step-change therapy, offering a more selective inhibition of T-type calcium channels.
- BTIG has raised its price target for Praxis to $843 from $507 per share while maintaining a ‘Buy’ rating.
Praxis Precision Medicines, Inc. (PRAX) announced on Monday that it has received a Breakthrough Therapy Designation (BTD) from the U.S. Food and Drug Administration (FDA) for its experimental drug, ulixacaltamide, aimed at treating essential tremor (ET).
The early clinical evidence suggested ulixacaltamide could provide meaningful improvement over current therapies for ET patients.
Expedited Regulatory Path
The designation, which speeds up both development and regulatory review for drugs addressing serious conditions, was granted after positive topline results from the Essential3 program, which included two Phase 3 studies of ulixacaltamide.
The BTD recognizes ulixacaltamide as a potential step-change therapy, offering a more selective inhibition of T-type calcium channels, a mechanism linked to abnormal neuronal signaling in essential tremor.
By targeting this pathway, Praxis aims to rebalance neuronal excitation and inhibition in patients, potentially improving motor function and daily life activities.
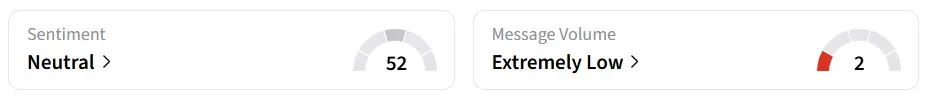
Following the update, Praxis Precision stock traded over 12% higher in Monday’s premarket. On Stocktwits, retail sentiment around the stock changed to ‘neutral’ from ‘bearish’ territory the previous day amid ‘extremely low’ message volume levels.
BTIG Raises Price Target
BTIG has raised its price target for Praxis to $843 from $507 per share while maintaining a ‘Buy’ rating. The firm cited the company’s upcoming launch of ulixacaltamide as “uniquely advantaged” in the essential tremor (ET) market.
BTIG emphasized that Praxis will develop an "unprecedented" database of one million ET patients, potentially supporting swift adoption of ulixacaltamide. The firm also raised its projected U.S. launch price for ulixacaltamide from $14,000 to $36,000, reflecting favorable pricing analogues.
PRAX stock has gained over 249% year-to-date.
Also See: After 80% Slide This Year, Solidion Draws A Second DOE Grant For Advanced Nuclear Tech
For updates and corrections, email newsroom[at]stocktwits[dot]com.









/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_1549425533_jpg_483afd6d69.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/unnamed_jpg_9dff551b50.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/large_stock_down_resized_901c19c371.jpg)
/filters:format(webp)https://news.stocktwits-cdn.com/Getty_Images_2235778544_jpg_2b7ceca102.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/Aashika_Suresh_Profile_Picture_jpg_2acd6f446c.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/large_hawaiian_electric_resized_4b766fd741.jpg)
/filters:format(webp)https://news.stocktwits-cdn.com/jaiveer_jpg_280ad67f36.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/large_sealsq_stock_market_representative_resized_b05435011f.jpg)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2230137825_jpg_d14459f501.webp)