Advertisement|Remove ads.
Why Did Vistagen Stock Tank 82% Today?

- PALISADE-3 aimed to assess acute anxiety relief during public speaking challenges.
- Participants received a single intranasal dose of fasedienol and were assessed during a simulated public speaking challenge.
- Lucid Capital downgraded Vistagen stock to ‘Neutral’ from ‘Buy’, and slashed its price target from $19 to $1.
Vistagen (VTGN) shares tanked 82% on Wednesday after the company announced that its PALISADE-3 Phase 3 trial for fasedienol in social anxiety disorder did not meet the primary endpoint.
The study aimed to assess intranasal fasedienol for acute anxiety relief during public speaking challenges.
Trial Design And Safety
The trial results showed no statistically significant difference between fasedienol and placebo in reducing anxiety, as measured by the Subjective Units of Distress Scale (SUDS). The least squares mean change from baseline was 13.6 ±1.54 for fasedienol versus 14.0 ±1.51 for placebo, a difference of 0.4.
PALISADE-3 was a randomized, double-blind, placebo-controlled U.S. multi-center study. Participants received a single intranasal dose of fasedienol and were assessed during a simulated public speaking challenge.
“We are disappointed by the unexpected results of this public speaking challenge trial, which are inconsistent with positive outcomes observed in Phase 2 and our PALISADE-2 Phase 3 study.”
-Shawn Singh, President and CEO, Vistagen.
How Did Stocktwits Users React?
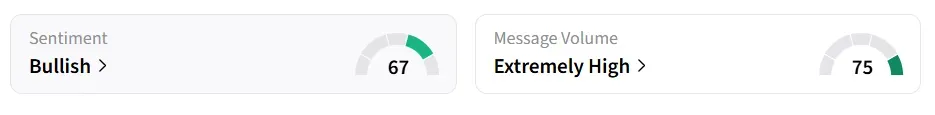
On Stocktwits, retail sentiment around Vistagen stock remained in ‘bullish’ territory, and message volume changed to ‘extremely high’ from ‘high’ levels in 24 hours.
A Stocktwits user said the drop in the stock may be exaggerated, considering the company has cash, other pipeline projects, and the treatment isn’t completely worthless.
Another user suggested waiting for PALISADE-4 results.
The company said it continues to investigate pherines as a potential new class of intranasal therapeutics targeting neurocircuitry.
Lucid Capital downgraded Vistagen stock to ‘Neutral’ from ‘Buy’, and slashed its price target from $19 to $1, citing the Phase 3 PALISADE-3 trial failure and uncertainty about the success of the ongoing PALISADE-4.
VTGN stock has declined by over 73% year-to-date.
Also See: Warner Bros. Discovery Rejects Paramount Bid And Stands Firm On Netflix Deal
For updates and corrections, email newsroom[at]stocktwits[dot]com.












/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2185274983_jpg_0354a0740b.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/Anushka_Basu_make_me_smile_in_the_picture_b92832aa_af59_4141_aacc_4180d2241ba8_1_2_png_1086e0ed8c.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_x_0d62438a6d.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Circle_Internet_jpg_add0182c9c.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2263898051_jpg_9e75888009.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2262920033_jpg_f596c67fd3.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_1227710498_jpg_fbb12d04bf.webp)