Advertisement|Remove ads.
KalVista Pharmaceuticals’ Hereditary Angioedema Drug Approved In EU, Switzerland: Retail Sees Stock Rallying To $22 In A Year

KalVista Pharmaceuticals (KALV) announced on Friday that the European Commission (EC) and the Swiss Agency for Therapeutic Products, Swissmedic, have approved its Ekterly for the symptomatic treatment of acute attacks of hereditary angioedema (HAE).
Ekterly was approved by both agencies for the treatment of adults and adolescents aged 12 years and older with HAE. It is the first and only oral on-demand treatment for HAE in the two regions, the company said.
Shares of the company traded lower by 2% at the time of writing. On Stocktwits, retail sentiment around KalVista stayed within ‘bullish’ territory over the past 24 hours, while message volume stayed within ‘normal’ levels.
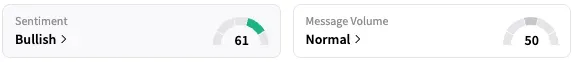
A Stocktwits user sees the stock rallying to $22 in 12 months.
Another opined that the stock has a lot of “potential and great setup.”
“With our U.S. launch progressing well and approvals now secured in the U.K., EU, and Switzerland, we look forward to bringing this innovation to more people living with HAE, beginning with our first European launch in Germany,” CEO Ben Palleiko said. The company expects to launch Ekterly in Germany in the fourth quarter and in Switzerland in the second half of 2026.
The recent approvals are based on the results of a late-stage trial where Ekterly achieved significantly faster symptom relief, reduction in attack severity, and attack resolution than placebo. The results also showed that the drug was well-tolerated, with a safety profile similar to that of the placebo.
The EC approval applies to all 27 EU member states as well as Iceland, Liechtenstein, and Norway. In August, the European Medicines Agency also granted Ekterly 10 years of market exclusivity.
Hereditary angioedema (HAE) is a rare genetic disease caused by a deficiency or dysfunction of a protein called the C1 esterase inhibitor, where patients experience painful and debilitating attacks of tissue swelling in various locations of the body that can be life-threatening depending on the area affected. Ekterly received approval from the U.S. Food and Drug Administration for the treatment of HAE in July.
KALV stock is up by about 61% this year and by about 24% over the past 12 months.
For updates and corrections, email newsroom[at]stocktwits[dot]com.











/filters:format(webp)https://news.stocktwits-cdn.com/Getty_Images_2202580632_jpg_9b97227b1a.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/Aashika_Suresh_Profile_Picture_jpg_2acd6f446c.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_ACHR_resized_jpg_25097dbec7.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/jaiveer_jpg_280ad67f36.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Plug_resized_jpg_82cf2f0bcd.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/astspacemobile_resized_jpg_8a6aa92413.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2231279747_jpg_9150b71435.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/Getty_Images_2250929484_jpg_8206df84ab.webp)