Advertisement|Remove ads.
Rocket Pharmaceuticals Stock Drops Pre-Market After FDA Pauses Gene Therapy Trial Over Patient Death: Retail Turns Bullish

Shares of Rocket Pharmaceuticals, Inc. (RCKT) traded 64% lower in Tuesday’s pre-market trading session after the U.S. Food and Drug Administration (FDA) placed a clinical hold on its trial related to investigational gene therapy for Danon disease called RP-A501, following the death of a patient.
The patient was part of the Phase 2 trial of RP-A501. They experienced an unexpected Serious Adverse Event (SAE) involving clinical complications related to a capillary leak syndrome (CLS), which led to their passing.
CLS is a condition where fluid and proteins leak out of the small blood vessels and into surrounding tissues, causing dangerously low blood pressure, fluid buildup in the body, and other complications.
Rocket Pharma said it is conducting a comprehensive root cause analysis and is in active dialogue with the FDA.
While the company voluntarily paused dosing in the study after the death of the patient, the FDA on May 23 also placed a clinical hold on the trial to allow further evaluation, the company said.
Rocket Pharmaceuticals said that it is working with the FDA to ensure the safety of all study patients and resume the trial “as expeditiously as possible,” but stopped short of providing a timeline for the resumption of trials.
The company also said that it expects its existing resources to be sufficient to fund operations into 2027. Rocket had cash, cash equivalents, and investments of $318.2 million at the end of the first quarter.
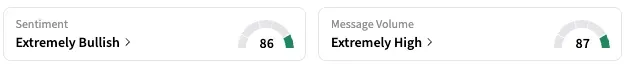
On Stocktwits, retail sentiment around Rocket Pharmaceuticals jumped from ‘bearish’ to ‘extremely bullish’ territory while message volume rose from ‘low’ to ‘extremely high’ levels.
A Stocktwits user opined that the drop in share price over the news could result in a strong entry point.
RCKT stock is down by over 50% this year and more than 70% over the past 12 months.
Read Next: Sanofi, VNVC Launch Vaccine Manufacturing Facility In Vietnam, Says Report: Retail’s Positive
For updates and corrections, email newsroom[at]stocktwits[dot]com.











/filters:format(webp)https://news.stocktwits-cdn.com/Getty_Images_2187726936_jpg_ca6aee809e.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/vivekkrishnanphotography_58_jpg_0e45f66a62.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_us_capitol_building_OG_jpg_388637a98c.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/Prabhjote_DP_67623a9828.jpg)
/filters:format(webp)https://news.stocktwits-cdn.com/Getty_Images_2250929484_jpg_8206df84ab.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/IMG_9209_1_d9c1acde92.jpeg)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_1163170868_jpg_3975bd8be2.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2247687123_1_jpg_5a8fc404b7.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/IMG_4530_jpeg_a09abb56e6.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_coinbase_new_jul_2eaf8eb2ac.webp)