Advertisement|Remove ads.
GSK, Spero Therapeutics Stop Antibiotic Trial For Complicated Urinary Tract Infections Early After Positive Results, Retail’s Thrilled

British pharmaceutical company GSK PLC (GSK) and its Massachusetts-based partner Spero Therapeutics (SPRO) said on Wednesday that they are stopping their late-stage trial evaluating the efficacy of its investigational oral treatment for complicated urinary tract infections (cUTI) earlier than expected following positive results.
The treatment, called Tebipenem Pivoxil Hydrobromide (HBr), was developed by GSK and Spero. In September 2022, GSK entered into an exclusive license agreement with Spero Therapeutics for the development and commercialisation of Tebipenem HBr in all markets, except certain Asian territories.
The early stop follows the trial meeting its primary goal of showing non-inferiority of Tebipenem HBr compared to an intravenous medicine in hospitalized adult patients with cUTI, including Pyelonephritis, where the infection spreads from the bladder to the kidneys.
The decision follows a recommendation from an Independent Data Monitoring Committee (IDMC), based on an analysis of data from 1,690 patients enrolled in the study. The committee did not identify any new safety concerns beyond what has been reported in other studies with Tebipenem, the companies said.
GSK said that it plans to work with U.S. regulatory authorities to include the data as part of a filing in the second half of the year.
If approved, Tebipenem HBr would be the first oral carbapenem antibiotic for patients in the U.S. who suffer from cUTIs, GSK said. An estimated 2.9 million cases of cUTIs are treated annually in the U.S. alone, the company noted.
The current standard of care for cUTIs includes carbapenem antibiotics, which are available for intravenous administration, requiring emergency department visits and hospitalization.
SPRO stock rocketed 203% on Wednesday morning following the news, while NYSE-listed shares of GSK fell marginally.
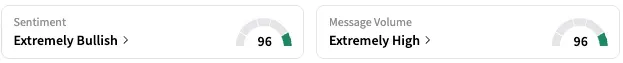
On Stocktwits, retail sentiment around SPRO jumped from ‘bearish’ to ‘extremely bullish’ territory over the past 24 hours while message volume rose from ‘low’ to ‘extremely high’ levels.
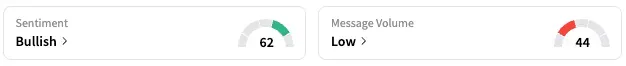
Meanwhile, retail sentiment around GSK rose from ‘neutral’ to ‘bullish’ territory, coupled with ‘low’ message volume.
SPRO stock is up by about 94% this year, while GSK shares are up by about 16%.
Read Next: Abercrombie & Fitch Stock Soars After Upbeat Q1 Results: Retail Turns More Bullish
For updates and corrections, email newsroom[at]stocktwits[dot]com.













/filters:format(webp)https://news.stocktwits-cdn.com/Johnson_and_Johnson_jpg_bc42927ca0.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/unnamed_jpg_9dff551b50.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/Getty_Images_2207639540_jpg_4ae2641504.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Jamie_Dimon_July_736ff90d31.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/Aashika_Suresh_Profile_Picture_jpg_2acd6f446c.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2250241035_jpg_937df85f43.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_kashkari_original_jpg_b7db42a385.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2199401088_jpg_656c1eacd4.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/vivekkrishnanphotography_58_jpg_0e45f66a62.webp)