Advertisement|Remove ads.
IBRX Stock Jumps 6% Pre-Market On BCG Update In Saudi Arabia – Retail Says Share Price Is ‘Attractive’

- ImmunityBio began discussions with SFDA to expand the use of ANKTIVA in combination with checkpoint inhibitors for multiple tumor types.
- ANKTIVA is already approved in Saudi Arabia for two indications: BCG-unresponsive non-muscle invasive bladder cancer and metastatic lung cancer.
- Earlier this year, ImmunityBio established a wholly owned local subsidiary to support regulatory filings, commercialization, and clinical development.
Shares of ImmunityBio, Inc. (IBRX) climbed about 6% in premarket trading on Tuesday after the Saudi Food and Drug Authority (SFDA) encouraged the company to submit a regulatory filing for its recombinant Bacillus Calmette-Guérin (rBCG), a move aimed at expanding treatment access in Saudi Arabia and easing the ongoing global shortage.
The company said it expects to file the submission within the next few weeks. BCG is an immunotherapy drug used to treat early-stage bladder cancer.
Expanding ANKTIVA Across Tumor Types
The company also began discussions with the SFDA to broaden the use of ANKTIVA in combination with checkpoint inhibitors for multiple tumor types beyond metastatic non-small cell lung cancer. ANKTIVA is an Interleukin-15 (IL-15) receptor agonist. IL-15 is an immune protein that helps develop and activate key cells that destroy cancer cells.
The therapy received accelerated approval in Saudi Arabia in January 2026 for patients whose disease progressed after standard treatments. Data from the QUILT-3.055 Phase 2b trial showed the combination restored checkpoint inhibitor activity across cancers such as melanoma, renal, gastric, and cervical tumors, with a median overall survival of 14.1 months in relapsed lung cancer patients.
Strengthening Regional Presence
ANKTIVA is already approved in Saudi Arabia for two indications: BCG-unresponsive non-muscle invasive bladder cancer and metastatic lung cancer. ImmunityBio has also established a wholly owned local subsidiary to support regulatory filings, commercialization, and clinical development across the Middle East and North Africa.
“Saudi Arabia was the first country in the world to grant approval for ANKTIVA in metastatic lung cancer. The QUILT-3.055 basket trial provides data that ANKTIVA rescues checkpoint activity across multiple tumor types, and we look forward to working with the SFDA to extend this approach to additional malignancies,” said Patrick Soon-Shiong, Executive Chairman and Global Chief Scientific and Medical Officer of ImmunityBio.
Soon-Shiong recently criticized the U.S. Food and Drug Administration (FDA), claiming the agency accelerated approval for Merck & Co.’s cancer therapy Keytruda while limiting ANKTIVA to a single bladder cancer indication.
Speaking on The Roger Stone Show, Soon-Shiong argued that regulatory delays are creating a bottleneck that could restrict patient access to a treatment he believes has significant life-saving potential.
How Did Stocktwits Users React?
Despite the premarket movement, retail sentiment for IBRX on Stocktwits remained ‘bearish’ over the past 24 hours.
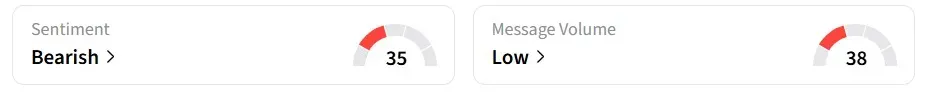
However, chatter was more bullish, with one user looking to buy more shares because the ‘price is attractive.’
Another user expects a strong rebound following a slight dip at market open.
The stock has seen strong buying interest so far this year, gaining more than 210%.
For updates and corrections, email newsroom[at]stocktwits[dot]com.










/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/large_stock_rising_resized_f17852d7aa.jpg)
/filters:format(webp)https://news.stocktwits-cdn.com/Aashika_Suresh_Profile_Picture_jpg_2acd6f446c.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2233918556_jpg_1c5248e175.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/Rounak_Author_Image_7607005b05.png)
/filters:format(webp)https://news.stocktwits-cdn.com/large_strait_of_hormuz_jpg_456f2fb6d3.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/vivekkrishnanphotography_58_jpg_0e45f66a62.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2259655311_jpg_20124bbeb9.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/Anushka_Basu_make_me_smile_in_the_picture_b92832aa_af59_4141_aacc_4180d2241ba8_1_2_png_1086e0ed8c.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_uniqure_jpg_33b6552285.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Sharp_Link_Gaming_jpg_60ce5684e3.webp)