Advertisement|Remove ads.
INMB Stock Gained 3% Today – What Did The FDA Say About Its Alzheimer’s Trial?
- The agency’s assessment was based on the company’s Phase 2 data package.
- The FDA supported the use of Clinical Dementia Rating Scale-Sum of Boxes, a scale used to determine dementia severity.
- INmune will enroll about 300 participants over nine months to confirm efficacy before expanding to a 1,000-patient Phase 3 trial.
INmune Bio, Inc (INMB) stock gave up some of its pre-market gains to trade 3% higher on Thursday morning, after the company said it has received official U.S. Food and Drug Administration (FDA) meeting minutes confirming regulatory alignment on its integrated Phase 2b/3 development plan for XPro1595, a potential therapy for early Alzheimer’s disease.
The FDA supported INmune Bio’s precision medicine strategy, which uses a trial design to identify patients whose inflammatory biomarker profiles are tied to soluble tumor necrosis factor (TNF) signalling, the treatment’s biological target.
The agency’s assessment was based on the company’s Phase 2 data package, which included cognitive and biomarker findings.
“XPro1595 represents a differentiated approach to Alzheimer’s disease, based on precision patient selection and selective immune modulation, with a favorable safety profile that included zero cases of ARIA in our Phase 2 study,” said David Moss, INmune Bio’s CEO.
FDA Approval Details
The FDA supported the use of the Clinical Dementia Rating Scale-Sum of Boxes (CDR-SB) as the primary endpoint, a scale used to assess dementia severity and track progression. It also did not object to INmune Bio’s plan to enroll patients identified by key inflammation-related biomarkers linked to the drug’s target.
The company said that the Phase 2b part will feature a nine-month evaluation period to build the clinical foundation for a subsequent Phase 3 study. INmune will enroll about 300 participants over nine months to confirm efficacy before expanding to a 1,000-patient Phase 3 trial lasting 18 months.
How Did Stocktwits Users React?
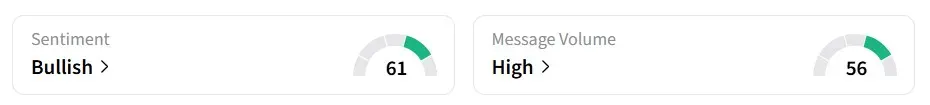
Retail sentiment on Stocktwits turned’ bullish’ from ‘neutral’ a day earlier, amid ‘high’ message volumes.
One user noted a tepid market reaction and said that it was an “excellent time to add” the stock to their portfolio.
Another user said the company was “moving in the right direction.”
INMB shares have declined around 0.6% so far this year.
Read also: Tesla’s China Retail Sales Slump 45% In January: Report
For updates and corrections, email newsroom[at]stocktwits[dot]com.











/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_1257075116_jpg_22f4f6802d.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/Anushka_Basu_make_me_smile_in_the_picture_b92832aa_af59_4141_aacc_4180d2241ba8_1_2_png_1086e0ed8c.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_tesla_robotaxi_jpg_ade25faaed.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/Rounak_Author_Image_7607005b05.png)
/filters:format(webp)https://news.stocktwits-cdn.com/Getty_Images_2221559761_jpg_71120b5aaa.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/shivani_photo_jpg_dd6e01afa4.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Core_Weave_jpg_861ba86dd3.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/vivekkrishnanphotography_58_jpg_0e45f66a62.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/Getty_Images_2235778544_jpg_2b7ceca102.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2243050664_jpg_37b52748e2.webp)