Advertisement|Remove ads.
Novo Nordisk Defends Alzheimer’s Trial Strategy For Semaglutide — Retail Warns A Pullback May Be Coming

- Novo Nordisk cited scientific evidence supporting its decision to study Semaglutide in Alzheimer’s disease.
- Initial Rybelsus trial findings will be presented this week, with full data due in March.
- The company discontinued the Alzheimer’s program after the late-stage studies missed their goals.
Novo Nordisk (NVO) reportedly defended its decision to test Semaglutide, its GLP-1 therapy, in Alzheimer’s disease on Tuesday, saying it launched pivotal trials in 2020 based on evidence from human studies, animal research and real-world findings.
The comments come after the company said last month that its late-stage studies did not confirm Semaglutide’s superiority over placebo in slowing disease progression and that it would discontinue the trials.
The stock closed the regular session down 2% at $47.43.
Speaking at the Clinical Trials in Alzheimer’s Disease meeting in San Diego, Peter Johannsen, Novo’s international medical vice president, acknowledged criticism that the studies had design flaws. He supposedly said the lack of statistically significant slowing of cognitive decline did not change the rationale for conducting them, calling it “a scientific question that needed an answer,” according to a Reuters report.
GLP-1 Activity In The Brain
Johannsen said data now consolidated on Novo’s website show evidence that the GLP-1 hormone is involved in neurotransmission, with multiple effects across the brain. He added that while Alzheimer’s is defined by toxic amyloid plaques, there remain major gaps in understanding the pathology. “This is a very complex disease with a lot of things going on with different genetic signatures,” he said, according to a Reuters report.
Novo will present initial findings on Wednesday from two two-year trials testing its GLP-1 pill Rybelsus against placebo in nearly 4,000 Alzheimer’s patients. Full results will be presented at another medical meeting in March. The company issued a statement last week confirming that the studies did not meet their goals.
What Real-World GLP-1 Data Really Shows
Johannsen pointed to real-world studies in diabetes patients that hinted at possible cognitive benefits from GLP-1 drugs, noting that some improvements appeared after about a year of use and continued to build over time. But he stressed that these findings need to be viewed carefully. Many of the analyses didn’t specify what type of dementia patients eventually developed, and some relied only on clinical impressions of Alzheimer’s rather than confirming the presence of amyloid plaques in the brain, the report said.
He added that real-world data can be skewed because patients who receive GLP-1 treatments often have better access to endocrinologists, rather than only primary care, and may be in higher socioeconomic groups than the general population. These point to factors that could delay when they seek help and, in turn, delay when a dementia diagnosis is made.
Stocktwits Traders Warn Of A Potential Slide
On Stocktwits, retail sentiment for Novo Nordisk was ‘bearish’ amid ‘low’ message volume.
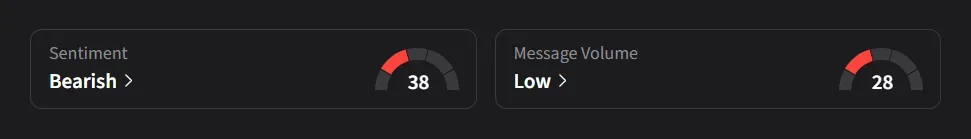
One user said the stock “will prob see $46.50 after the usual 3-4AM dump tomorrow.”
Meanwhile, another user called the stock “terrible.”
Novo Nordisk’s U.S.-listed stock has declined 44% so far this year.
For updates and corrections, email newsroom[at]stocktwits[dot]com.









/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2263454468_jpg_23f4595a31.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/Anushka_Basu_make_me_smile_in_the_picture_b92832aa_af59_4141_aacc_4180d2241ba8_1_2_png_1086e0ed8c.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2264020227_jpg_4d7420bef3.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2259210190_jpg_d48bbe3269.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_1396534113_jpg_b0e09f299b.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Jane_Street_3ac3fb6443.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Truth_social_5bfbc7389b.webp)