Advertisement|Remove ads.
Pfizer, BioNTech’s Updated COVID-19 Vaccine Shows Robust Immune Response In Late-Stage Study – More Details Inside
Pfizer Inc. (PFE) and BioNTech SE (BNTX) announced on Monday that their updated COVID-19 vaccine elicited a robust immune response in a late-stage trial among adults aged 65 and older, as well as those aged 18 through 64 with at least one underlying risk condition.
The trial was aimed at evaluating the safety, tolerability, and immunogenicity of a 30-µg dose of the updated vaccine. Preliminary data showed a robust increase in neutralizing antibodies targeting the LP.8.1 sublineage of the COVID-19-causing virus following vaccination, the companies said.
It added that after 14 days of vaccination, LP.8.1-neutralizing antibody titers exceeded pre-vaccination levels, on average, by at least 4-fold in both age groups. The study enrolled 100 participants.
While PFE stock traded 2% lower at the time of writing, BNTX stock fell 7%. On Stocktwits, retail sentiment around PFE stock stayed within ‘bearish’ territory over the past 24 hours, while message volume stayed at ‘low’ levels.

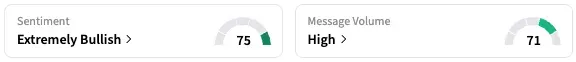
Meanwhile, sentiment around BNTX stayed at ‘extremely bullish’ levels, while retail chatter remained ‘high’.
A Stocktwits user expressed surprise at PFE stock trading lower after the news.
The companies stated that the data is intended to reinforce previous findings from vaccine studies and is not meant to replace the post-marketing commitments requested by the U.S. Food and Drug Administration. The safety profile of the vaccine was consistent with previous studies in the trial, with no new safety concerns identified, they added.
The updated vaccine received FDA approval last month. To date, five billion doses of the Pfizer-BioNTech COVID-19 vaccine have been distributed globally.
While PFE stock is down about 8% this year, BNTX stock is down over 8%.
For updates and corrections, email newsroom[at]stocktwits[dot]com.














/filters:format(webp)https://news.stocktwits-cdn.com/large_peter_thiel_OG_jpg_9d74d987ca.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/Prabhjote_DP_67623a9828.jpg)
/filters:format(webp)https://news.stocktwits-cdn.com/large_LUV_Southwest_bags_jpg_0c65f623c2.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/IMG_4530_jpeg_a09abb56e6.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_micahel_saylor_OG_3_jpg_4f304c479d.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_rivian_automotive_jpg_e356c1abe5.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/IMG_8805_JPG_6768aaedc3.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2252871865_jpg_74865c27a7.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/IMG_9209_1_d9c1acde92.jpeg)
/filters:format(webp)https://news.stocktwits-cdn.com/large_immunitybio_jpg_eb6402d336.webp)