Advertisement|Remove ads.
SNTI Shares Surge Over 20% In Pre-Market Trade After FDA RMAT Designation For Cancer Treatment

- SENTI-202, the immune cell therapy cancer treatment, received a Regenerative Medicine Advanced Therapy (RMAT) designation that would make it eligible for quicker reviews.
- This is the second FDA designation for the treatment this year.
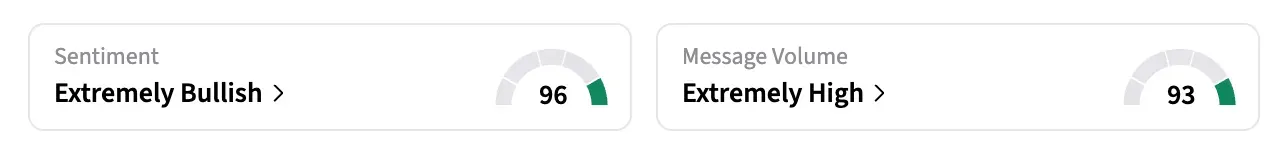
- On Stocktwits, retail sentiment for the stock rose to ‘extremely bullish’ territory while message volume jumped to ‘extremely high’ levels at the time of writing.
Shares of Senti Biosciences, Inc. (SNTI) surged over 20% on Tuesday’s premarket trading after the company announced positive news around its cancer treatment.
The clinical-stage biotech company announced in a press release that the U.S. Food and Drug Administration (FDA) gave SENTI-202, an immune cell therapy cancer treatment, a Regenerative Medicine Advanced Therapy (RMAT) designation.
What Does This Mean?
The RMAT designation from the FDA for the company’s cancer treatment indicates an expedited development and review, providing benefits similar to the FDA’s Breakthrough Therapy and the Fast Track programs such as higher interactions with the Agency through the development process as well as eligibility for quicker and even priority reviews.
“This significant FDA designation validates both the tremendous need for better treatments for R/R AML and the promise of SENTI-202 to transform the therapeutic landscape for this notoriously aggressive cancer,” said Timothy Lu, Co-Founder and CEO of Senti Biosciences, in the press release. “We are incredibly pleased with the exciting clinical progress we recently shared at the ASH conference on SENTI-202.”
Data from its ongoing trial showed a 50% overall response rate and 42% complete remission at the recommended Phase 2 dose, the company revealed. Earlier this year, the U.S. FDA granted the treatment Orphan Drug Designation.
How Did Stocktwits Users React?
On Stocktwits, retail sentiment around SNTI jumped from ‘neutral’ to ‘extremely bullish’ territory over the past 24 hours, while message volume rose from ‘normal’ to ‘extremely high’ levels.
One user on the platform predicted that STNI’s share price could go up to $5. The stock was trading around $2.85 at the time of writing.
Shares of SNTI have fallen over 37% year to date.
For updates and corrections, email newsroom[at]stocktwits[dot]com











/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_1396534113_jpg_b0e09f299b.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/Anushka_Basu_make_me_smile_in_the_picture_b92832aa_af59_4141_aacc_4180d2241ba8_1_2_png_1086e0ed8c.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Jane_Street_3ac3fb6443.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Truth_social_5bfbc7389b.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2221283194_jpg_8178c730a4.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/large_Morgan_Stanley_HQ_logo_5431c7f2ad.jpg)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_1549425533_jpg_483afd6d69.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/unnamed_jpg_9dff551b50.webp)