Advertisement|Remove ads.
TRAW Stock Slides In Volatile Session – What’s The Latest On Its Phase 2 Trial For COVID-19 Treatment?
- The open-label trial enrolled 90 patients and compared Ratutrelvir with Paxlovid, an oral antiviral medication from Pfizer.
- Early data analysis supports Ratutrelvir’s favorable tolerability profile, faster symptom resolution, and lack of viral rebound events, the company said.
- Traws is advancing Tivoxavir Marboxil as a potential once-monthly oral preventive treatment for seasonal flu.
Shares of Traws Pharma, Inc. (TRAW) gained about 5% in premarket trading but slipped after the opening bell following the company’s announcement that it has completed enrollment in its Phase 2 trial of Ratutrelvir, an investigational oral antiviral for mild-to-moderate COVID-19.
The open-label trial enrolled 90 patients and compared Ratutrelvir with Paxlovid, an oral antiviral medication from Pfizer, used to treat mild-to-moderate COVID-19. Traws said that patients unable to take Paxlovid are often at greater risk of severe disease, and that Ratutrelvir may help address this unmet need as an alternative treatment option.
Patients receiving Ratutrelvir were treated with a once-daily oral dose for 10 days, while those in the separate treatment arm received standard Paxlovid dosing for five days. According to the company, early data analysis supports Ratutrelvir’s favorable tolerability profile, faster symptom resolution, and lack of viral rebound events.
“The combination of early and sustained symptom improvement, extended dosing duration, absence of viral rebound observed to date, and favorable tolerability supports the strategic hypothesis that Ratutrelvir may have utility in reducing post-acute sequelae of SARS-CoV-2 infection (Long COVID),” said Robert R. Redfield, Chief Medical Officer of Traws Pharma.
Influenza Treatment Update
In addition to the COVID-19 program, Traws provided an update on Tivoxavir Marboxil, an investigational antiviral for influenza. The company is advancing the drug as a potential once-monthly oral preventive treatment for seasonal flu, expanding its pipeline of therapies targeting respiratory viral diseases.
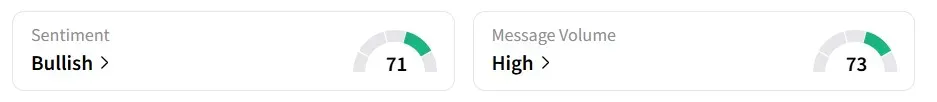
Meanwhile, retail sentiment for TRAW remained in the ‘bullish’ territory over the past 24 hours, amid ‘high’ message volumes.
The stock has rebounded sharply in 2026, with TRAW up more than 115% amid renewed buying interest.
Also See: Why Did MNKD Stock Jump Over 7% In Pre-Market Today?
For updates and corrections, email newsroom[at]stocktwits[dot]com.











/filters:format(webp)https://news.stocktwits-cdn.com/large_Circle_Internet_jpg_add0182c9c.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/Anushka_Basu_make_me_smile_in_the_picture_b92832aa_af59_4141_aacc_4180d2241ba8_1_2_png_1086e0ed8c.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2263898051_jpg_9e75888009.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2262920033_jpg_f596c67fd3.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_1227710498_jpg_fbb12d04bf.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2233918556_jpg_1c5248e175.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2227669377_jpg_9a115c3623.webp)