Advertisement|Remove ads.
Watch Out, Novo Nordisk & Eli Lilly: Chinese Biotech Steps Into the Ring To Take On Global Weight-Loss Leaders
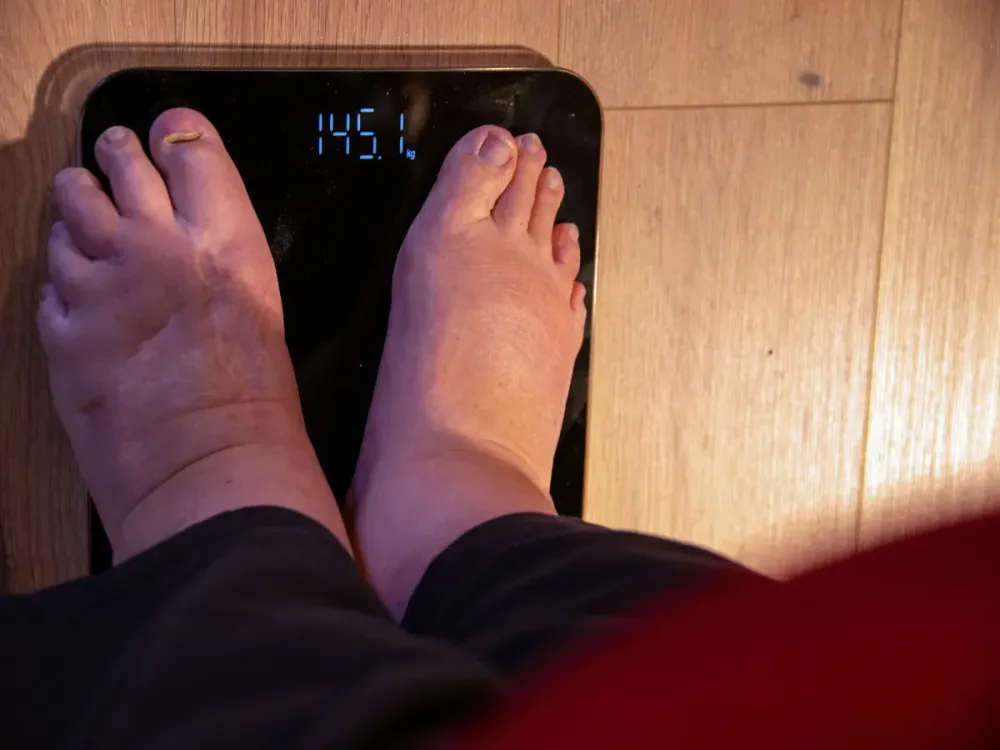
Chinese biotech firm Hangzhou Sciwind Biosciences is stepping into the high-stakes global obesity drug race, with its experimental treatment ecnoglutide helping patients shed more than 15% of their body weight over 48 weeks in a late-stage clinical trial.
Ecnoglutide, a once-weekly GLP-1 receptor agonist, is under regulatory review in China for obesity and diabetes.
According to a Bloomberg report citing CEO Hai Pan, approval could come as early as 2026.
The results, unveiled at the American Diabetes Association conference and published in The Lancet, suggest that the drug’s effect is comparable to Eli Lilly’s Zepbound in similar China-based trials.
Pan said about 93% of patients on the highest dose of ecnoglutide lost at least 5% of their body weight, surpassing comparable response rates reported for both Zepbound and Novo Nordisk’s Wegovy in China.
Although the trial did not directly compare ecnoglutide to these competitors, Sciwind believes the results put its drug on par with leading global GLP-1-based therapies.
Ecnoglutide, which mimics GLP-1, is also being tested in Australia and New Zealand, and has been out-licensed to a partner in South Korea.
Pan said the company is in early talks with potential collaborators to expand development beyond China.
Tricia Tan, a professor at Imperial College London, said in a Lancet commentary that the data are likely generalizable and called ecnoglutide a “viable competitor” in the GLP-1 analogue space, adding that broader access to these therapies could be accelerated through new entrants like Sciwind.
Sciwind expects to secure over 10% of the Chinese obesity drug market, which it estimates to be worth more than 100 billion yuan ($13.9 billion).
The firm is also advancing multiple candidates for metabolic conditions, three of which were recently out-licensed to UK-based Verdiva Bio, backed by $411 million in funding.
For updates and corrections, email newsroom[at]stocktwits[dot]com.









/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_1813801150_jpg_9e452258fa.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/Anushka_Basu_make_me_smile_in_the_picture_b92832aa_af59_4141_aacc_4180d2241ba8_1_2_png_1086e0ed8c.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2190302521_jpg_796f64970e.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2194612888_1_jpg_5f7b7f6186.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_1384896168_jpg_87fab3f04d.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2263571605_jpg_f769289486.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2233719278_jpg_46dfac21ee.webp)