Advertisement|Remove ads.
Axsome Advances Toward FDA Filing For Drug To Treat Narcolepsy

- Axsome expects to make NDA submission by January 2026.
- AXS-12 has also received Orphan Drug Designation for narcolepsy.
- The designation could provide Axsome up to seven years of U.S. market exclusivity.
Axsome Therapeutics (AXSM) on Wednesday said it received formal pre–New Drug Application (NDA) meeting minutes from the U.S. Food and Drug Administration that support an NDA submission for AXS-12 for the treatment of narcolepsy.
AXS-12, also called reboxetine, is being developed to treat cataplexy in narcolepsy by targeting norepinephrine and dopamine activity in the brain. Cataplexy involves sudden and brief muscle weakness.
Following the FDA’s feedback, Axsome said its regulatory data package is sufficient to support an NDA submission, which the company expects to complete in January 2026. The AXS-12 development program includes three controlled efficacy trials and a completed long-term safety study.
AXS-12 has also received Orphan Drug Designation for narcolepsy. The designation could provide up to seven years of U.S. market exclusivity and a waiver of FDA application user fees if the drug is approved, the company said.
“We are pleased with the FDA pre-NDA meeting minutes, which allow completion of the NDA submission for AXS-12 for the treatment of cataplexy in patients with narcolepsy shortly in January 2026,” said Herriot Tabuteau, Chief Executive Officer of Axsome.
AXSM shares were up 1.5% in premarket trading on Wednesday. It was also among the top trending tickers on Stocktwits at the time of writing.
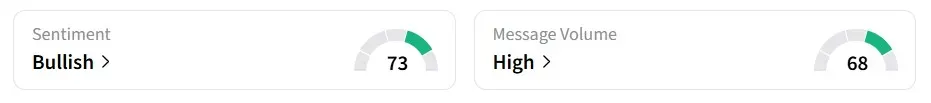
How Did Stockwits Users React?
Retail sentiment on Stocktwits flipped to 'bullish' from 'bearish,' amid 'high' message volumes.
One user was bullish about the developments on two fronts.
Another user expects the stock to rise to $200.
Year-to-date, the stock has surged over 100%.
Read also: One Chinese EV Stock Surged 80% In 2025 – But Two Others Told Very Different Stories
For updates and corrections, email newsroom[at]stocktwits[dot]com.









/filters:format(webp)https://news.stocktwits-cdn.com/large_us_stocks_war_jpg_f2a208ae56.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/IMG_8805_JPG_6768aaedc3.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2263711678_jpg_7dcbe85e4f.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/IMG_4530_jpeg_a09abb56e6.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_bitcoin_2026_jpg_f51342601b.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/Prabhjote_DP_67623a9828.jpg)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2202237165_jpg_188d67bdb5.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/IMG_9209_1_d9c1acde92.jpeg)
/filters:format(webp)https://news.stocktwits-cdn.com/large_us_iran_2_jpg_0c6789db95.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2263736058_jpg_2b8f901978.webp)